Atoms have very little mass. So instead of using their mass in grams or kilograms (can you imagine being the scientist trying to put an individual atom on the scales?!) all atoms have a relative atomic mass.
Remember seeing the little numbers on the periodic table next to an element's symbol? They are the mass number and atomic number.

When we refer to relative atomic mass, we basically mean the mass number of an atom of that element.
So if we wanted to work out the relative formula mass for a substance such as carbon dioxide, CO2, we first need to know the relative atomic mass of carbon and oxygen.
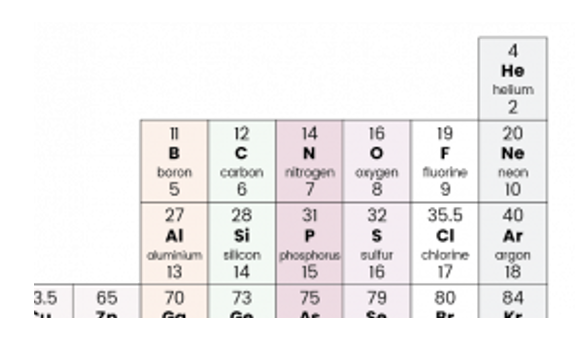
As you can see carbon has a mass of 12 and oxygen has a mass of 16.
To calculate the relative formula mass for a substance:
1) Work out how many atoms of each element there are in the chemical formula
2) Add together the mass values for all the atoms of each element present
So for CO2 there is one atom of carbon and two atoms of oxygen so the relative formula mass of carbon dioxide would be:
12 + 16 + 16 = 44
Note: There are no units for relative formula mass.
This does look tricky, but it will get easier if we try some questions.

Let's have a go at calculating the relative formula mass of various substances.







