All matter can be subdivided into various categories. See the diagram below.
Matter is either classed as pure or impure. As you can see, the two types of pure substances are elements and compounds.
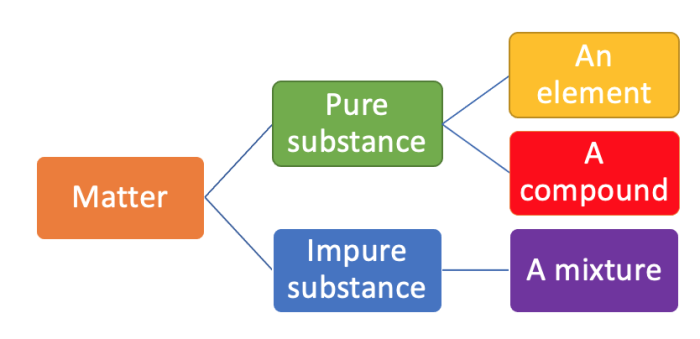
Pure substances are made up of only one type of element or one type of compound. As soon as there is even one molecule of a different element or compound in a pure substance, it turns it into an impure substance.
So, if you drop a cube of sugar into a beaker of water, once the sugar particles have dissolved and mixed with the water molecules, it is an impure substance.

The composition of a pure substance is pretty uniform throughout - so if you took a small sample of gold from a large block, you would see that it has the same composition no matter from where you took the sample.
.png)
But if you took a sample of impure pond water, its composition may vary depending on where you took the sample. In some samples, you might see water molecules, some salt ions and a few microorganisms. If you took a sample slightly deeper down, you might see no microorganisms but some molecules of sand with the water and salt ions.

Pure substances cannot be separated into anything simpler without undergoing a chemical reaction. Physical changes will not alter the chemical composition of a pure substance either - for example a glass of pure water would still be pure once it is frozen or if it evaporated.
Impure substances consist of different kinds of substances (they can be elements or compounds) physically combined, but not chemically combined. That means that they can sometimes be separated easily by physical separation, such as filtering.
Think you can distinguish between pure substances and mixtures? Let's try some questions!








