Substances can change states - from solid to liquid to gas, and back again. This usually happens when they are heated or cooled.
Energy plays a big role in changes of state.
Let's find out more.
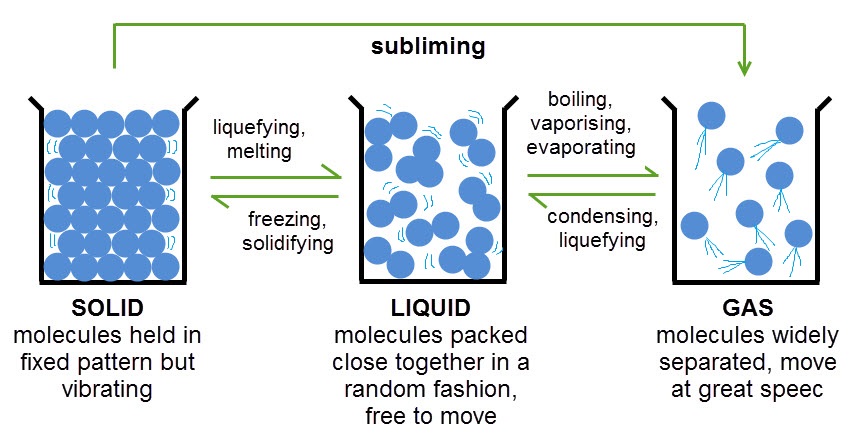
As you can see, when heated, particles in a substance start to move around more and get further apart - the more they are heated the further apart they get and eventually the substance will change state. This is because the particles are absorbing energy from their surroundings. The opposite happens when a substance is cooled. The particles slow down and become closer together because they are transferring energy to the surroundings.
There are some important words in the diagram above that you'll need to remember about state changes.
Freezing and condensing are the words we use to describe changes of state when energy is released.
Boiling, evaporating, melting and subliming are the words we use to describe changes of state when energy is absorbed.
You've probably come across most of these before. One that you may not have come across before is subliming. Sublimation happens when a solid is heated and changes directly into a gas, skipping the liquid state altogether. This is what happens to solid carbon dioxide, or 'dry ice' as it is known.
Got it? Let's try some questions on heating and cooling and the resulting changes of state.
.jpg)







