What happens to your ice cream on a really hot day, and what happens to the water vapour in the air as it touches a cold window?

That's right, they change state. The particles in the ice cream and the water vapour start behaving differently as they are heated or cooled. Let's find out more.
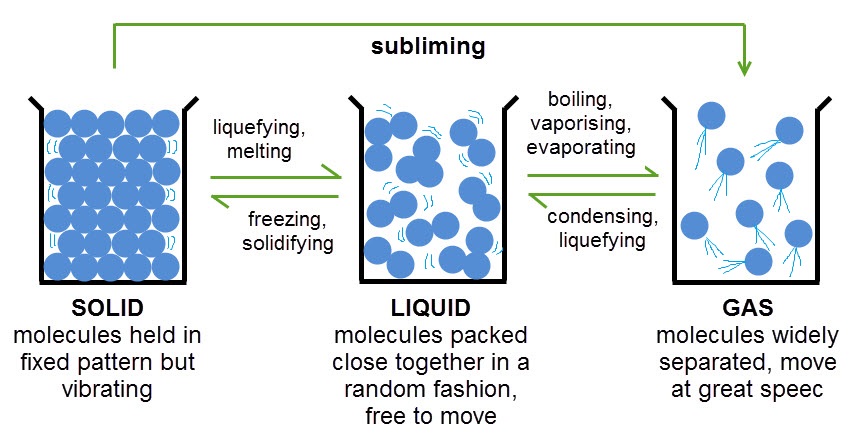
As you can see, when heated, particles in a substance start to move around more and get further apart - the more they are heated the further apart they get and eventually the substance will change state. This is because the particles are gaining energy. The opposite happens when a substance is cooled. The particles slow down and become closer together because they are losing energy.
There are some important words in the diagram above that you'll need to remember about state changes. One that you may not have come across before is subliming. Sublimation happens when a solid is heated and changes directly into a gas, skipping the liquid state altogether. This is what happens to solid carbon dioxide, or 'dry ice' as it is known.
Got it? Let's try some questions on heating and cooling and the resulting changes of state.








