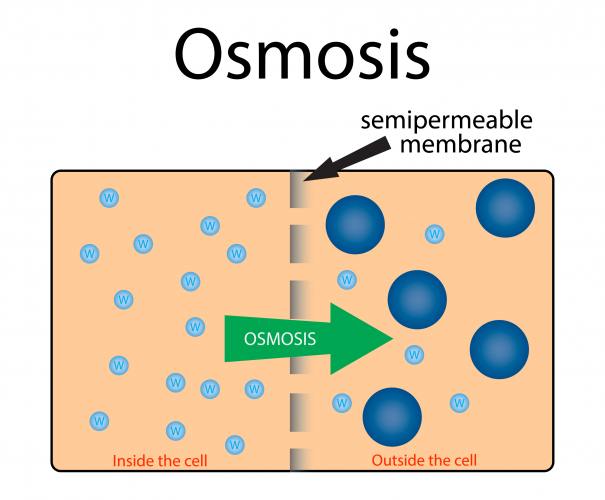
Osmosis is the diffusion of water molecules from a low concentration solution to a high concentration solution, across a partially permeable membrane.
A partially permeable membrane has holes or pores in it that allow water molecules through, but are too small to allow larger molecules through.
During osmosis, water molecules diffuse from pure water or dilute solution to more concentrated solutions.
Dilute solutions have a high concentration of water molecules.
Concentrated solutions have a low concentration of water molecules.
Osmosis in plants

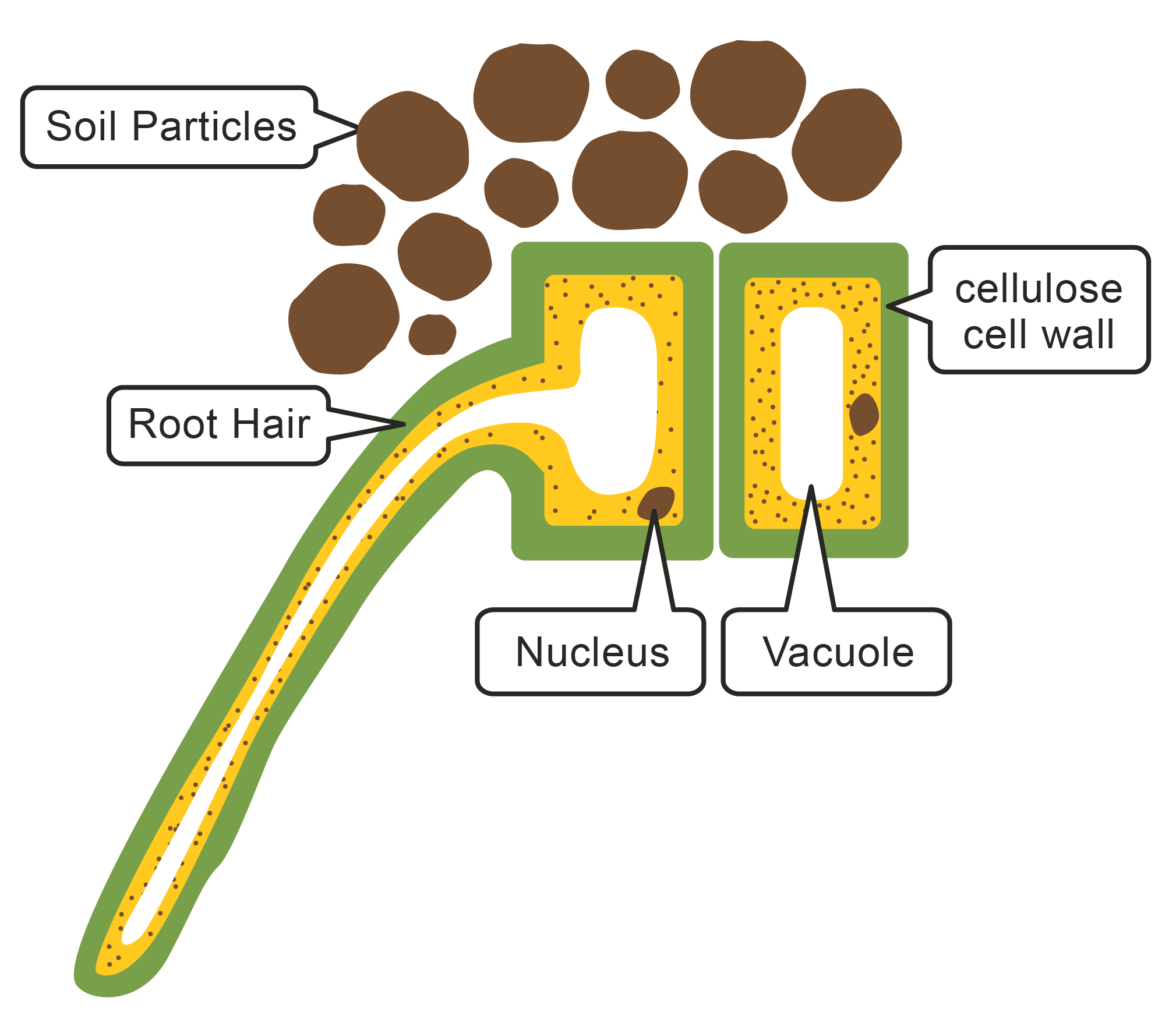
Plant cells are surrounded by a partially permeable cell membrane. This allows water and other small molecules to diffuse across. Plant cells have a strong cell wall surrounding the membrane, which offers support and protection.
Plants require water in order to photosynthesise. The roots of a plant contain root hair cells, which are specialised cells that increase the surface area for maximum absorption of water by osmosis. In pure water, plant cells will take in water via osmosis and become firm or turgid. In a concentrated solution (not much water present), the cell loses water and starts to shrink and becomes flaccid.
Calculations Involving Osmosis

Osmosis can be demonstrated using cubes of potatoes of roughly the same mass. By placing the cubes in different concentrations of sugar solutions, the cubes might gain or lose mass, or might even stay the same mass.
Scientists will be able to calculate the change in mass to see how much mass was gained or lost by using the following equation:
Final mass (g) – initial mass (g) = change in mass (g)
In the following activity, you will define and describe osmosis.








