Group 1 of the periodic table contains some of the strangest elements there are. They are metals, but not as you know them…
The Group 1 elements are also called the alkali metals. They sit in the left hand column of the periodic table. The first three reading down are lithium, sodium and potassium. They're the most important ones. The other elements in Group 1 are rubidium, caesium and francium.
Like most metals, they are shiny and grey. They turn dull grey in a few seconds when they are exposed to air because they react very quickly with oxygen. Most metals react with oxygen - the strange thing about alkali metals is how fast the reaction is. The reactions follow this pattern:
lithium + oxygen → lithium oxide
sodium + oxygen → sodium oxide
potassium + oxygen → potassium oxide
They conduct electricity, but nobody ever uses alkali metals to make electrical wires.
So what’s strange about them? Firstly, some of them have a very low density - lithium, sodium and potassium all float in water, so they must be less dense than water. Secondly, they are very soft - you can cut them with a knife or scalpel, as if they were a piece of cheese. As you go down Group 1, the metals become even softer.
The strangest thing about the alkali metals is what happens when you put them in water. All the alkali metals react very vigorously with water:
lithium + water → lithium hydroxide + hydrogen
sodium + water → sodium hydroxide + hydrogen
potassium + water → potassium hydroxide + hydrogen
The hydroxide solutions made in this reaction turn the water alkaline - that’s why these metals are called alkali metals.
As you go down Group 1, this reaction becomes more energetic: lithium fizzes, sodium fizzes a lot and potassium burns with a characteristic lilac (purple) flame. We can see that as you go down Group 1, the elements become more reactive.
A scientist once put a piece of caesium into a glass dish of water, and the reaction released so much energy that the dish shattered instantly.
The reason that the alkali metals behave in this way is linked to the arrangement of electrons in shells. Let’s look at sodium as an example. Sodium is atomic number 11, so a sodium atom has 11 electrons. That’s 2 in the first shell, 8 in the second shell, and one in the third shell. We can write that as 2.8.1, or draw a picture like this:
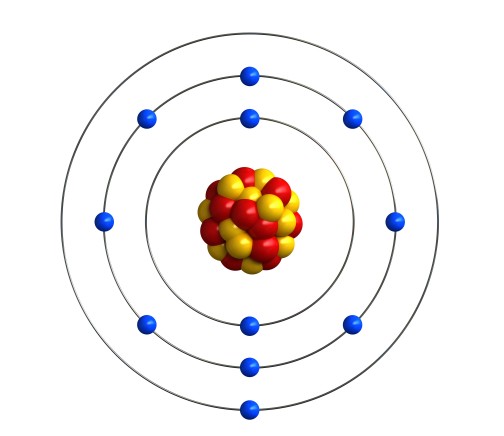
In fact, all the alkali metals have 1 electron in their outermost shell. Lithium is atomic number 3, so its electron configuration is 2.1. Potassium is atomic number 19, which is 2.8.8.1. The same goes for the heavier alkali metals. That single electron in the outermost shell is very unstable. All the alkali metals need to lose their outermost electron. Since there is only one electron to lose, it can happen fairly easily, and this process releases lots of energy.
Now let's move on to some questions.








