Ever wanted to take a bath in oil? Well, in Azerbaijan oil is so important to their economy that you literally could take a bath in it! Crude oil is one of the most important natural resources, making everything from medicine to the petrol you put in your car. It is finite, which means it will eventually run out, and non-renewable as it cannot be made again. It is a fossil fuel, like coal and natural gas.
Fossil fuels were made over millions of years from the remains of dead organisms that were compressed together while trapped in the Earth. Dead animals made oil and dead plants made coal.
Crude oil is a mixture of hydrocarbons (substances made from carbon and hydrogen only). In order to separate it into its components, it needs to be heated through a process called fractional distillation. Here is a diagram that shows how crude oil is separated:
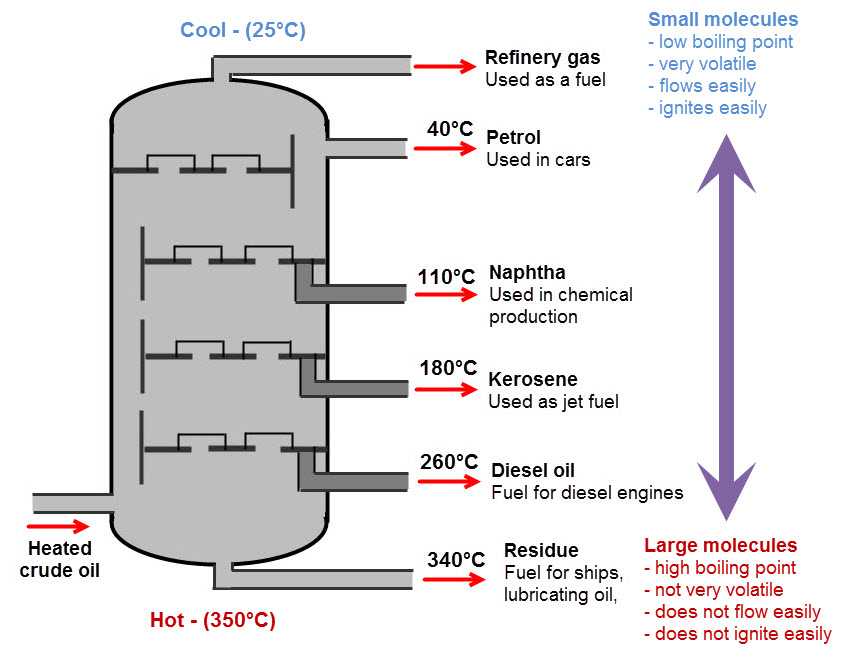
You need to know this diagram and be able to talk about how the properties of the hydrocarbons change over the different fractions.
The component hydrocarbon molecules are long chains held together by intermolecular forces. The forces are broken while boiling.
Lighter molecules with shorter chains need less energy for the forces to be broken, so they are easily separated. They have low boiling points. One example is petrol.
Heavy molecules, like bitumen, have longer chains and more energy is needed to separate them. They have high boiling points.
As the heat in the fractional distillation tower increases, small molecules evaporate first and rise to the top, while heavier ones stay at the bottom.
The smaller chains are also able to flow over each other better, meaning that they will be less viscous (they will flow more easily). The longer the chains, the harder it is for them to flow over each other making them more viscous.
Have another look at the diagram above until you are sure you understand it, and then move on to give the questions a try.








