During chemical reactions, energy transfer takes place to and from the surroundings. This transfer is in the form of thermal energy, i.e. heat. A chemical reaction can be either exothermic or endothermic.
Exothermic Reactions
Exo- means 'out '- think about words like 'exit'.
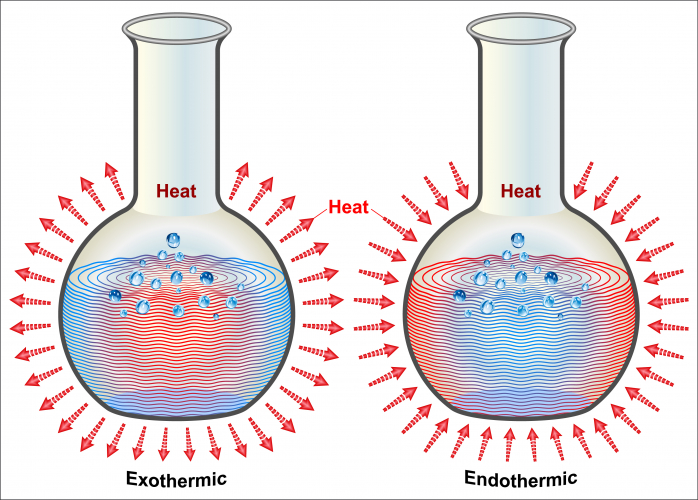
Exothermic reactions release heat to their surroundings. The picture shows an example of an exothermic reaction:

Clearly, this looks like an explosion. Explosions are exothermic reactions, but a reaction can be exothermic without exploding. Any increase in temperature indicates an exothermic reaction.
If you were to hold a container when an exothermic reaction were taking place, you would feel the heat, and a thermometer inside the container would show an increase in temperature.
Examples of exothermic reactions are combustion (burning) and neutralisation.
Hand warming pads contain chemicals which undergo an exothermic reaction when pressed together.
Endothermic Reactions
Endo- means 'inside' - an endoscope is a camera that a doctor puts inside a patient's body. Endothermic reactions take in energy from their surroundings.
If you were to hold a container when an endothermic reaction were taking place, you would feel your hands getting cold, as the reaction would take heat from the container, and even your hands, if you kept holding it! A thermometer in the container would show a decrease in temperature.
The energy of the products is higher than the energy of the reactants.
Examples of endothermic reactions are electrolysis, photosynthesis and thermal decomposition. In all of these reactions, we need to keep putting energy into the reaction for it to keep going. In electrolysis the energy source is the battery, in photosynthesis the energy is light, and in thermal decomposition the energy source is a heat source (like a flame).
Cold pads to relieve injuries can use chemicals which undergo an endothermic reaction.
Reaction profiles
A reaction profile is a sort of graph comparing the energy of a set of chemicals before, during and after a chemical reaction. Before the reaction is on the left, and after the reaction is on the right. Energy is on the vertical axis - high energy is high up and low energy is low down.
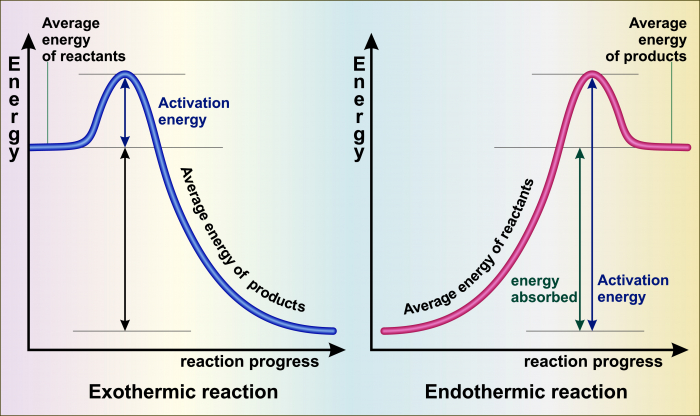
In an exothermic reaction (on the left), the energy of the chemicals starts high and ends lower. The energy difference is released into the surroundings.
In an endothermic reaction (on the right), the energy of the chemicals starts low and ends higher. The energy difference is taken from the surroundings.
Both reaction profiles have a peak in the middle, where the energy of the chemicals is highest of all. This is because nearly all chemical reactions need some energy input so that they can begin to happen.
The energy needed to start a chemical reaction is called the activation energy - it's the energy needed to make the reaction active.
Reaction profiles help us to visualise the difference between exothermic and endothermic reactions, and to compare reactions involving different amounts of energy - for example, an explosion releases a large amount of energy in a short time.
Now it's time for some questions on this.








