Acids and alkalis are both types of chemical that you wouldn't want to spill on your skin, but each of them is able to make the other one disappear. What's going on?
All chemicals fit on a scale called the pH scale. This is a number scale going from 0 (very acidic) to 14 (very alkaline). 7 is in the middle, so that is the pH of pure water, called neutral.

There are some patterns in this scale - acidic things (pH less than 7) often taste sour (think of lemon juice - citric acid, vinegar - acetic acid, or stomach acid - hydrochloric acid). Alkaline things are often used for cleaning (think of soap, or bleach). The more extreme the pH is, the more dangerous the chemical.
The rainbow pattern in the picture above shows you one way of measuring the pH of a chemical, using universal indicator. We can have universal indicator as a liquid, or we can make paper with universal indicator soaked into it. If we add this to some chemical, the colour of the indicator will change to indicate the pH of the chemical.
For example, we could put a few drops of lemon juice onto universal indicator paper. The paper would turn orange where the drops were, which would show us that the pH of the juice was about 2.
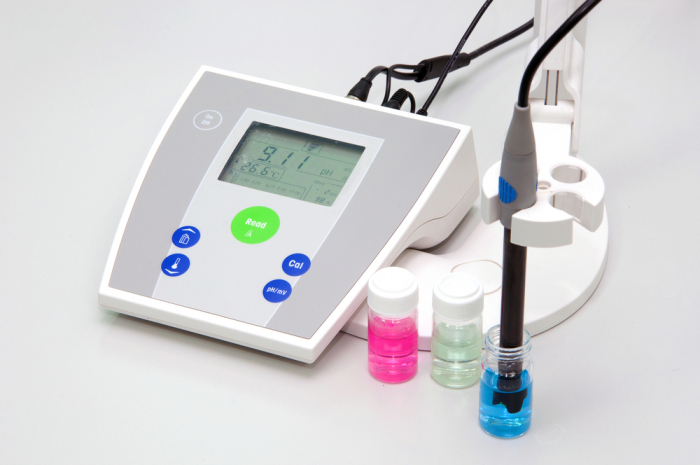
Universal indicator isn't very precise, but it gives us a rough idea of the pH of a solution. We can get much more accurate results using an electronic pH meter.
The thing that determines whether a solution is acidic or alkaline is the presence of H+ ions or OH- ions.
Acidic solutions have excess H+ ions dissolved in them. Alkaline solutions have excess OH- ions dissolved in them.
Both these particles are very reactive, which is why both acids and alkalis are potentially harmful. However, if H+ and OH- combine, they make H2O, which is water. That's why we can use acid to neutralise alkali, and alkali to neutralise acid.
Let's have a go at some questions now.








