Ammonia is an important industrial product. It is manufactured using the Haber process on a large scale in huge factories. Ammonia can then be used to make fertilisers, explosives and dyes.

The equations for the reaction are shown below
Nitrogen + Hydrogen ⇌ Ammonia
N2(g) + 3H2(g) ⇌ 2NH3(g)
To make ammonia, nitrogen and hydrogen are pumped through pipes into a compressor. The compressor then increases the pressure that the gases are under and also raises the temperature, bringing the particles closer together and more likely to bump into each other, ultimately resulting in more successful collisions.
The gases then enter a reactor containing an iron catalyst. The catalyst further speeds up the rate of reaction, as its presence means that there are even more successful collisions between the particles of gases. The product, ammonia, is then cooled and leaves the system. Any unreacted nitrogen and hydrogen are recycled, and go round again to be turned into ammonia.
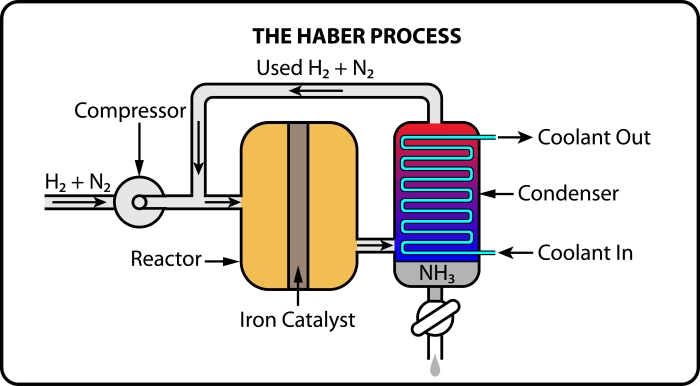
The presence of the iron catalyst in the Haber process also means that a slightly lower temperature can be used, which is cheaper in the long run.
Let's have a go at some questions on the Haber process now.
You can look back at this page at any point by clicking on the red help button on the screen.








