Have you ever rubbed a balloon on your hair to build up a static charge on it?

Were you able to stick the balloon to the wall? The balloon was 'charged-up' but the wall was neutral. Why did they stick? Let's find out!
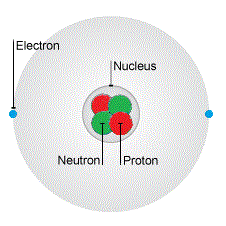
Here is a quick reminder of the causes of static electricity - it all starts with the atom. Atoms have positive protons and neutral neutrons in their small nucleus, and negative electrons orbit that nucleus.
When two insulators rub together, friction can cause electrons to move from one of the insulators to the other, building up a static charge. This is what causes static electricity.
When you rub a balloon on your hair, the friction causes electrons in your hair to move onto the balloon's surface.
The balloon has gained electrons, so it is slightly negatively charged.
Your hair has lost electrons, so it is slightly positively charged.
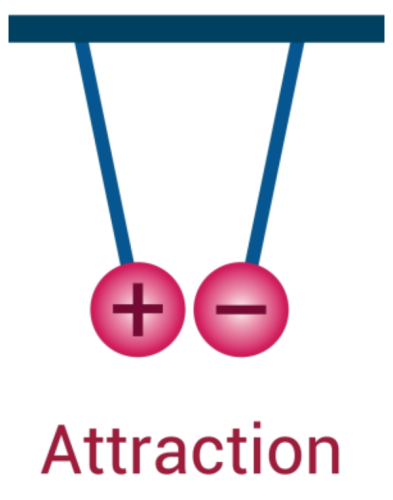
This causes your hair to stand on end because your positively charged hair is attracted to the negatively charged balloon.
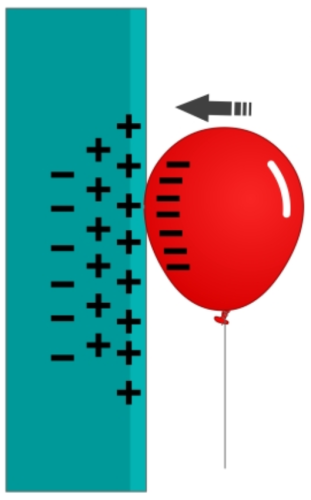
So, why does the negatively charged balloon stick to a neutral wall?
It's a result of a concept we call polarisation of charge.
When we put the balloon on the wall, the negative electrons in the balloon, repel the electrons that make up the atoms in the surface of the wall.
The electrons can be repelled because they are not in fixed positions in the atom - external forces can cause them to move. The electrons move away from the negatively charged balloon.
The nucleus of an atom is positively charged because it contains neutral neutrons and positive protons. The nucleus is in a fixed position and cannot move.
What this means is that, as the negative electrons move away, positive nuclei are left behind.
Overall, the wall is still neutral, but the surface is slightly positively charged and the area behind the top layer is slightly negatively charged. This is called polarisation of charge.
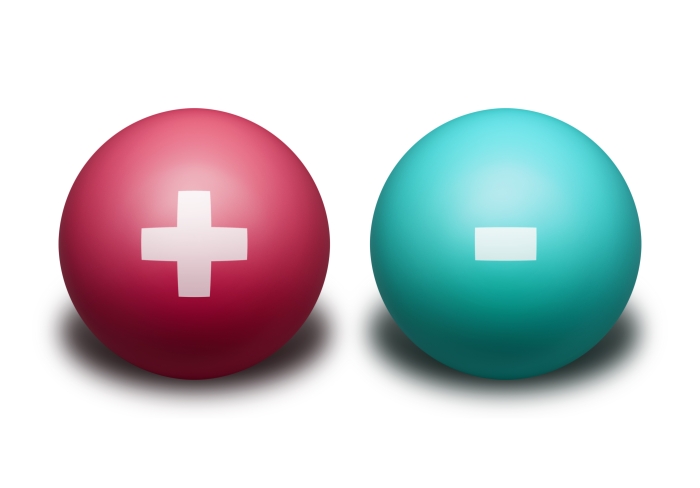
What if we tried to stick a positively charged object to a neutral surface? What do you think would happen?
The positively charged object would still be attracted to the neutral surface!
The positively charged object, when close to the surface, would attract the electrons in that surface, pulling them towards the front.
The front of the surface would now be slightly negative, and the back would be slightly positive. The positively charged object will be attracted to the surface!
This is another example of polarisation of charge - when an object is overall neutral, but there is a movement of some of the charges in that object, leaving regions of positive and negative charges.
Now that we understand these ideas, let's try some questions!