Solids, liquids and gases. These are called the three states of matter.
Ice, steam and water - they are all made up of the same compound, but quite different in their properties.
.jpg)


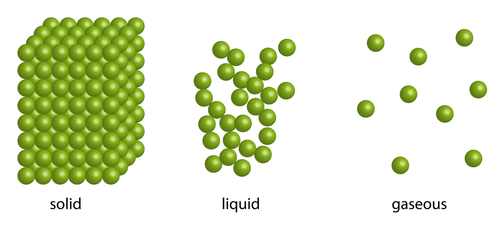
Although all are made out of water particles, the solid, liquid and gaseous states of water have quite different properties. This is because of the way their particles are arranged.
In a solid, the tiny particles that make up the solid are very close together in a neat regular arrangement, all touching each other and moving only by vibrating on the spot. They cannot move much at all as there are strong forces of attraction between the particles, which explains why solids have a fixed shape.

The particles in a liquid are arranged in a more random way. They are close together, most of them touching each other, but there are some small gaps. The particles also have enough energy to break free of some of the forces of attraction between the particles. So the particles can move around a little, over each other, allowing liquids to flow and be poured. This is the reason why liquids take the shape of the container they are in.

The particles in a gas are spaced very far apart, move around very fast and they are randomly arranged. This is because the particles have lots of energy so can overcome the forces of attraction between the particles. Because of this, gases can be easily compressed, or squashed - they have no fixed shape or volume.
Can you use your knowledge of the three states of matter to answer some questions? Great, here we go.......







