When a chemical reaction takes place, there are several changes that may occur. These visible changes are called the indicators of a chemical reaction. There are several indicators of reaction:
Colour Change

A change in colour is a sign of a chemical reaction. For example, frying an egg is a chemical reaction with a colour change - colourless to white.
Gas Given Off

During a chemical reaction, a gas can be produced. This can be seen by bubbles forming in the reaction or by a coloured gas being produced (such as smoke).
Solid Formed

If two solutions are mixed and a solid is formed, this is called a precipitate. A precipitate is an insoluble solid formed by reacting two liquids. For example, when milk goes very sour, a solid is formed.
Temperature Change

An energy change involves a change in temperature (either up or down). For example, the burning of a match involves a release of both heat and light.
In a chemical reaction, one or more new substances are always produced. So it is difficult to reverse a chemical reaction, as atoms have been rearranged.
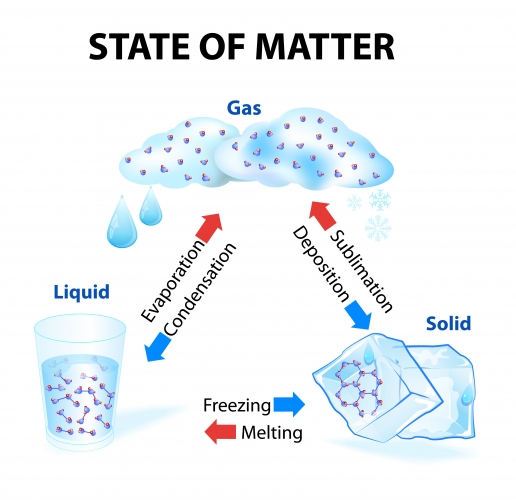
So what is a physical change?
A physical change is where a substance just changes state. Physical changes are reversible - we always end up with the same substance we started with but sometimes in a different state.
Let's have a go at some questions now.