In a chemical reaction, one or more new substances are produced. Atoms are rearranged during a chemical reaction, but the number of atoms does not change.
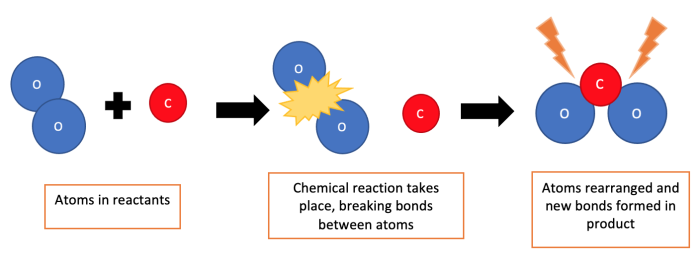
It is therefore difficult to reverse a chemical reaction because the atoms have been rearranged.
When a chemical reaction takes place, there can be several changes that occur. The following visible changes can be used as evidence that a chemical reaction has taken place.
Colour Change

Gas Given Off

Solid Formed

Temperature Change

However, just because we may see these, it doesn't necessarily mean that a chemical reaction has occurred.
A kettle produces bubbles as its water is boiling, but there's not a chemical reaction going on inside the kettle.
Similarly, when you make a cup of tea, there is a colour change in the water from colourless to brown, but there has not been a chemical reaction.

The observations described in the two examples above are caused by a physical change, rather than a chemical change.
So what is a physical change?
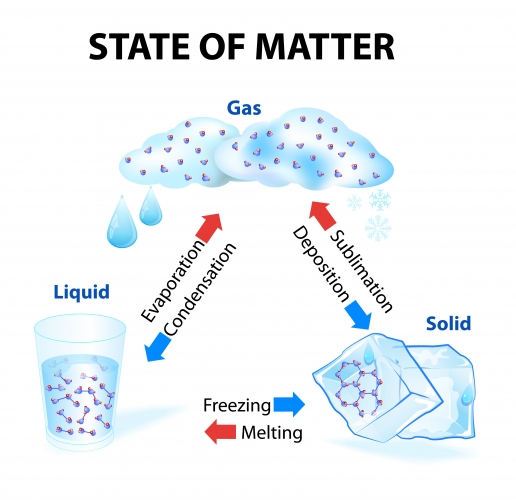
A physical change is where a substance just changes state. Physical changes are reversible - we always end up with the same substance we started with but sometimes in a different state.
Let's have a go at some questions now.








