When particles collide, they bump off each other in different directions. Over time, these collisions cause the particles to spread out. The name given to this process of spreading out is diffusion.
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. That means that they will spread out from where there are a lot of them, to where there are fewer of them. The difference between the area of high concentration and area of low concentration is called a concentration gradient.
Diffusion takes place down a concentration gradient.
The way particles are arranged in a substance and how much energy they have will determine how well diffusion takes place through that substance.
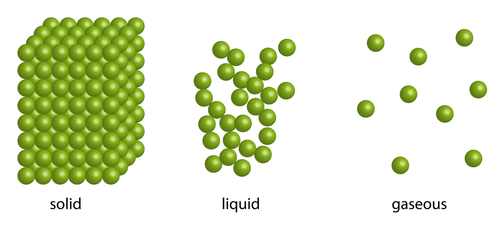
Diffusion can't really happen in solids, but can take place in liquids. Diffusion happens really quickly in gases as gas particles are spaced far apart and also because they bump into each other more often, sending particles of substances flying in all directions, helping them to spread out!
Temperature affects diffusion. The hotter a substance is, the faster diffusion will happen. This is because the particles in the substance will have more energy and so will move faster at higher temperatures. Similarly, if you reduce the temperature of a substance, diffusion will happen slower. This is because when particles are colder, they have less energy and therefore move around less and much slower.
Have you got your head around all that?

Great! Then let's take a look at a few questions.








