Acids are shown on the pH scale. Strong acids have a pH of 1-3 and weaker acids have a pH of 4-6.
Acids react with metals to produce a salt and hydrogen gas. This can be shown in a word equation:
Acid + Metal ![]() Salt + Hydrogen
Salt + Hydrogen
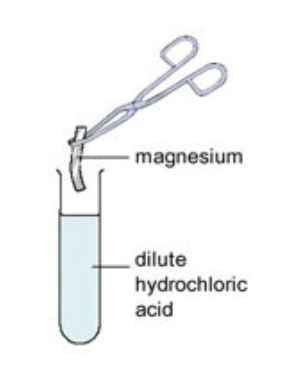
Not all metals react with acids - unreactive metals such as gold and platinum do not react with acids, but metals such as magnesium and zinc react very vigorously with acids. Some metals, such as sodium, are so reactive that they should never be placed into acid.
When a small piece of magnesium metal is added to hydrochloric acid, lots of bubbles of hydrogen gas are produced and the conical flask becomes hot. The hydrogen gas can be collected and tested.
But how can we tell what gas has been created - how do we know for certain it is hydrogen? Well, we can perform a gas test. There are three tests that show us whether the gas produced is hydrogen, carbon dioxide or oxygen.
Gas tests
Hydrogen gas is explosive and when a small volume is collected and ignited a 'squeaky' pop is produced. This is the test for hydrogen gas, but this should only be performed with small volumes of hydrogen gas as large volumes will result in large explosions.
We can test for carbon dioxide gas using limewater. When carbon dioxide is bubbled through limewater, it turns cloudy white.

We can test for oxygen gas by using a glowing splint. The gas is collected in a test tube and a glowing splint is inserted into it. If the splint relights, then oxygen is present.
These three tests are very important to remember for all future chemistry work!
So remember, acids react with metals to produce a salt and hydrogen gas. Strong acids will react much more violently than weak acids.
Acid + Metal ![]() Salt + Hydrogen
Salt + Hydrogen
Let's move on to some questions now.