Acids react with metal carbonates such as calcium carbonate to produce a salt, water and carbon dioxide. This is a neutralisation reaction. This reaction can be shown in a word equation:
acid + metal carbonate ![]() salt + water + carbon dioxide
salt + water + carbon dioxide
Calcium carbonate is found in rocks such as limestone, chalk and marble. These substances have been used for many years to make buildings and statues. For example, shown here is a picture of the Leshan Giant Buddah in China that was carved from limestone rock over one thousand years ago.

The statue is slowly being damaged by acid rain. The acidic rain is reacting with the limestone it is made from, slowly eroding the statue.
This reaction can be shown in the lab. If a small piece of calcium carbonate (remember, limestone is mainly calcium carbonate) is added to hydrochloric acid it begins to fizz because of the carbon dioxide gas being produced. The piece of calcium carbonate will become smaller until there is no more left and the reaction will stop.
The carbon dioxide gas produced can be identified using a substance called limewater. Limewater becomes cloudy white in the presence of carbon dioxide gas.
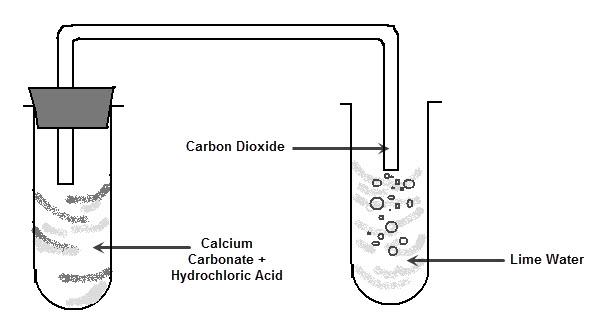
This reaction occurs when any metal carbonate is added to an acid.
Calcium carbonate, along with some other carbonates, is also added to indigestion remedies to neutralise stomach acid. That's because metal carbonates are alkalis. We know that when acids and alkalis are mixed they create a neutral solution of pH 7 - a neutralisation reaction.
Let's try some questions on this.








