Ice, steam and water - they are all made up of the same stuff!
.jpg)


Although they are all are made out of water particles, the solid, liquid and gaseous states of water have quite different properties. This is because of the way their particles are arranged, the amount of energy they have and whether this energy is enough to overcome the strong forces of attraction that normally hold particles together.
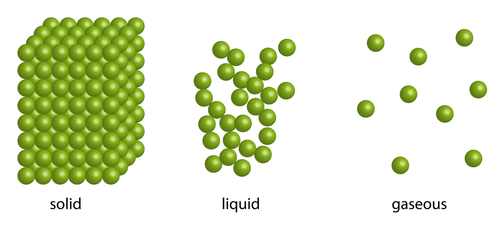
In a solid, the tiny particles that make up the solid are very close together in a neat regular arrangement, all touching each other and moving only by vibrating on the spot. They cannot move much at all as there are strong forces of attraction between the particles, which explains why solids have a fixed shape.
The particles in a solid don't have much energy. They don't have enough energy to break the forces between them. This is why solids have the properties they do. What do you think might happen if you heated a solid very gently? What would happen to the particles in the solid? Well, by heating the solid gently, you would give the particles a bit more energy. This would cause them to move around a little more and move slightly further apart. Not enough to turn into a liquid, but just enough to make the solid expand a little.
The particles in a liquid are arranged in a more random way. They are close together, most of them touching each other, but there are some small gaps. The particles also have more energy than in solids. So the particles can move around a little, over each other, allowing liquids to flow and be poured. This is the reason why liquids take the shape of the container they are in.
The particles are able to move around more than in a solid as they have more energy. The more energy particles have, the more easily they can break the strong forces that normally hold them together, so they can get further apart the more energy they have.

The particles in a gas are spaced very far apart, move around very fast and they are randomly arranged. Gases can be easily compressed, or squashed - they have no fixed shape or volume. Gas particles have lots of energy. So much energy in fact that they can easily overcome the strong forces trying to hold them together, meaning they can spread out. It's almost as if the gas particles want to be as far away from each other as they can, so they will carry on moving and spreading out until the walls of a container stops them. Pressure in gases is caused by particles colliding with the walls of the container they are in, and exerting a force on these walls. This is called gas pressure.
If a gas is heated slightly, the energy of the gas particles increases. These higher energy particles move faster, colliding harder and more often with each other and the walls of the container. This increases the pressure. We can also increase the pressure of a gas by squashing the container. When you decrease the volume of a sealed container containing a gas, the particles collide with the walls more often, increasing the pressure.

Can you use your knowledge of the three states of matter to answer some questions? Great, here we go.......







