Metals have special properties that make them very useful, such as electrical conductivity, however, not all metals have the same properties.
The properties of metals are a result of how their atoms are arranged. For example, all metals are solids at room temperature (except for mercury, which is a liquid).
Listed below are some of the properties of metals.
Electrical Conductivity
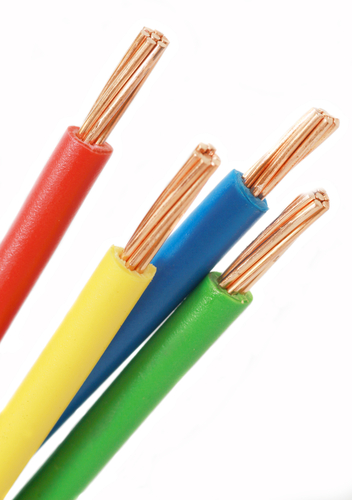
All metals conduct electricity, which makes them essential in the manufacture of all electrical equipment. Manganese has the lowest electrical conductivity, but all metals do conduct electricity. Copper is a good conductor and is used in most electrical wiring.
Thermal Conductivity

Thermal conductivity is the ability of a metal to allow heat to pass through it. All metals conduct heat but as with electrical conductivity some metals are better at it than others. Copper is also an excellent conductor of heat but bismuth has the lowest thermal conductivity of all the metals.
High Melting Points

Most metals have high melting points and that is why they are found as solids at room temperature. However, as always, there are some exceptions. Mercury is a metal with a low melting point, which means that it is a liquid at room temperature. This property makes mercury a great material to use in thermometers.
Malleability

Malleability is the ability of a substance to be bent or rolled into sheets. Most metals are malleable which makes them ideal to bend into all sorts of shapes from poles to sheets. Antimony is a brittle metal and will break if it is bent or rolled.
Strength
.jpg)
Most metals are strong and this makes them an excellent building material for constructing bridges, buildings, cars etc., but, as always, there are some exceptions. The metal sodium is a soft metal that can be cut very easily.
Magnetic
Some metals, such as, iron, cobalt and nickel are magnetic which means they are attracted to magnets or can be turned into a magnet. Not all metals are magnetic.
Let's have a go at some questions now.








