From all your work, and your life experience, you'll know that things around you are basically found in three different states:
Solid Liquid and Gas
You also know that pure substances can move between these states according to whether they're being heated or cooled. Yes?
 |
 |
 |
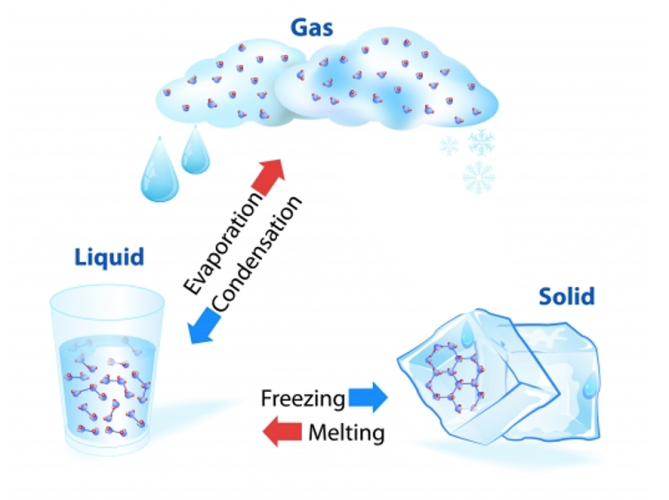
Now, look at this diagram carefully - it shows how materials change between solids, liquids and gases:
| melting | evaporation | |||
| SOLID | LIQUID | GAS | ||
| freezing | condensation |
Now look at it again...
What's happening to change a solid to a liquid to a gas? It's being heated.
What's happening to change a gas to a liquid to a solid? It's being cooled.
These are physical changes and they can be reversed, which means they go back to what they were before.
When materials change in this way, it's called changing state.
Do you notice each special word as the state changes (melting, condensing, etc.)?
Water is the most obvious example to demonstrate how it changes state.
Solid = Ice
Liquid = Water
Gas = Steam (or, better, water vapour).
Of course, many substances you come across will change like this - water's just an obvious example.
OK, to be able to talk about and describe these changes, we must understand the science vocabulary associated with each of the physical changes, so that's what we're going to be looking at in this activity.

Are you ready to get involved? Let's get going!
Remember, you can come back to this page at any time simply by clicking the red button on the right-hand side of any page you're on.