John Dalton created a model in the 1800s where he laid down some key ideas about atoms which we still use in science to this day. However, further scientists over the years have discovered new things about the atom, which has caused us to change our thinking slightly from Dalton's simple atomic model.
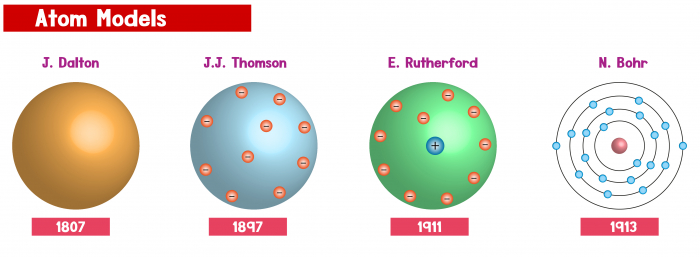
It was discovered that there were actually even smaller subatomic particles inside an atom - these are electrons, protons and neutrons.
It was found that in the very middle of an atom, there is an area called the nucleus, and this is where the protons and neutrons are. And on the very outside of the atom, the electrons circle around the nucleus, like planets around the sun!
The different subatomic particles also have different charges. Neutrons are neutral, which means they don't have a charge. They're in the nucleus, in the middle of the atom. The protons are positive and they are also in the nucleus, and they're what gives the nucleus its positive charge. The electrons are negatively charged, and whizz around the outside in shells.
Woah, that's a lot of new information.

Shall we take a look at a few questions about this?
You can always return to this page if you need to by clicking on the red help button on the screen.







