Mixtures contain different substances which are fairly easy to separate.
There are various ways in which you can separate a mixture.
Filtration
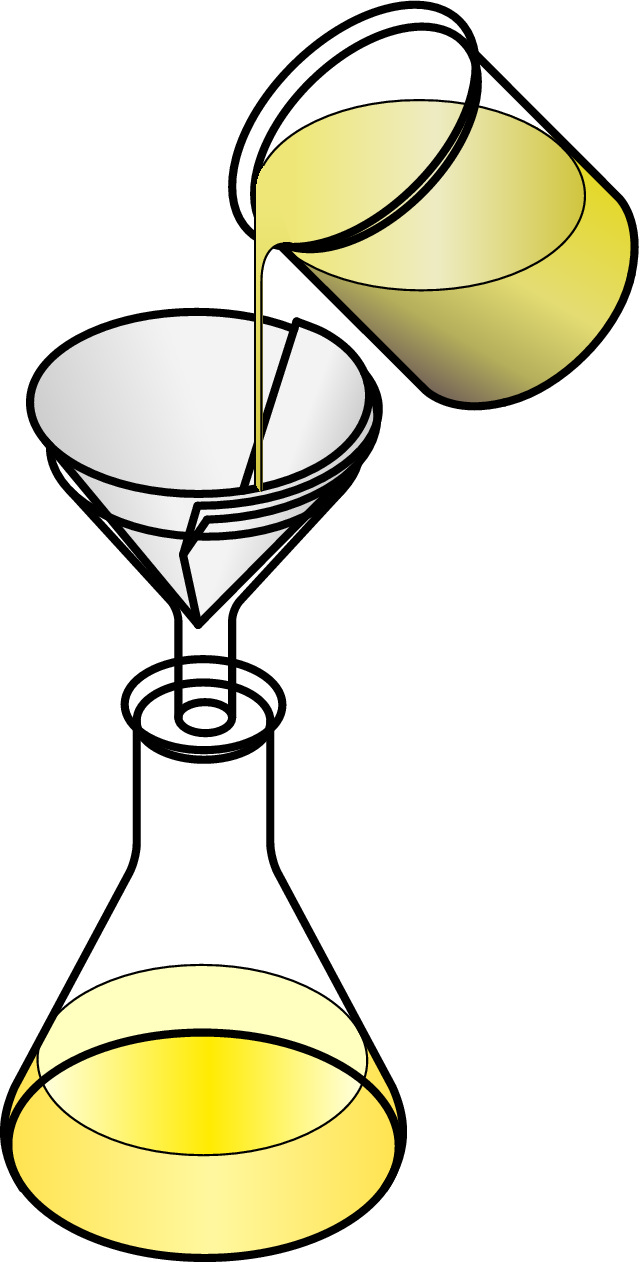
A mixture containing an insoluble solid in a liquid such as sand in water, can be separated by using filtering. The mixture is poured through the filter funnel that is lined with filter paper, and the water can go straight through but the sand is trapped in the filter paper.
Evaporation
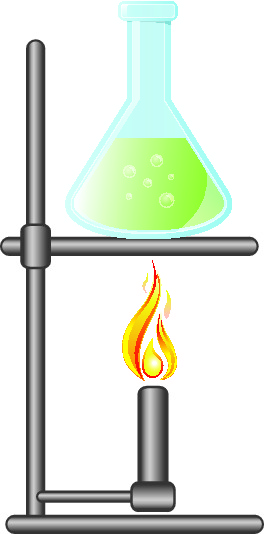
A mixture containing a soluble substance in a liquid such as salt and water, will require evaporation. This is where the solution is left overnight for the water to slowly warm up and turn into a gas, or the solution can be heated to make the process faster. The water evaporates and the salt is left behind.
Distillation
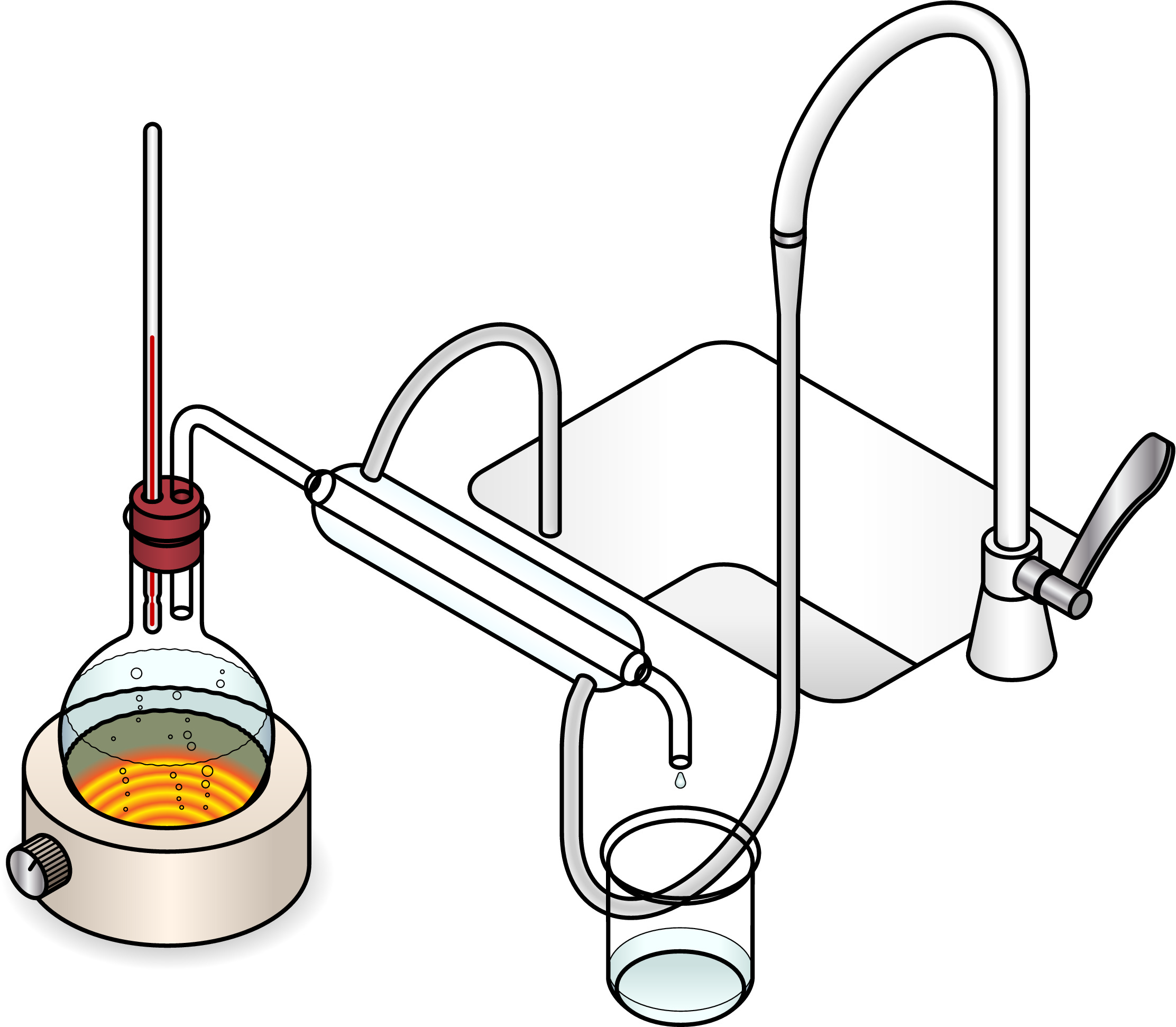
Distillation is a separation technique used to separate a liquid from a mixture. For example, water can be separated from salt solution by distillation. Distillation involves boiling the mixture and then condensing the gas to produce a liquid.
Chromatography
Sometimes, a number of different substances can be dissolved in a solution.
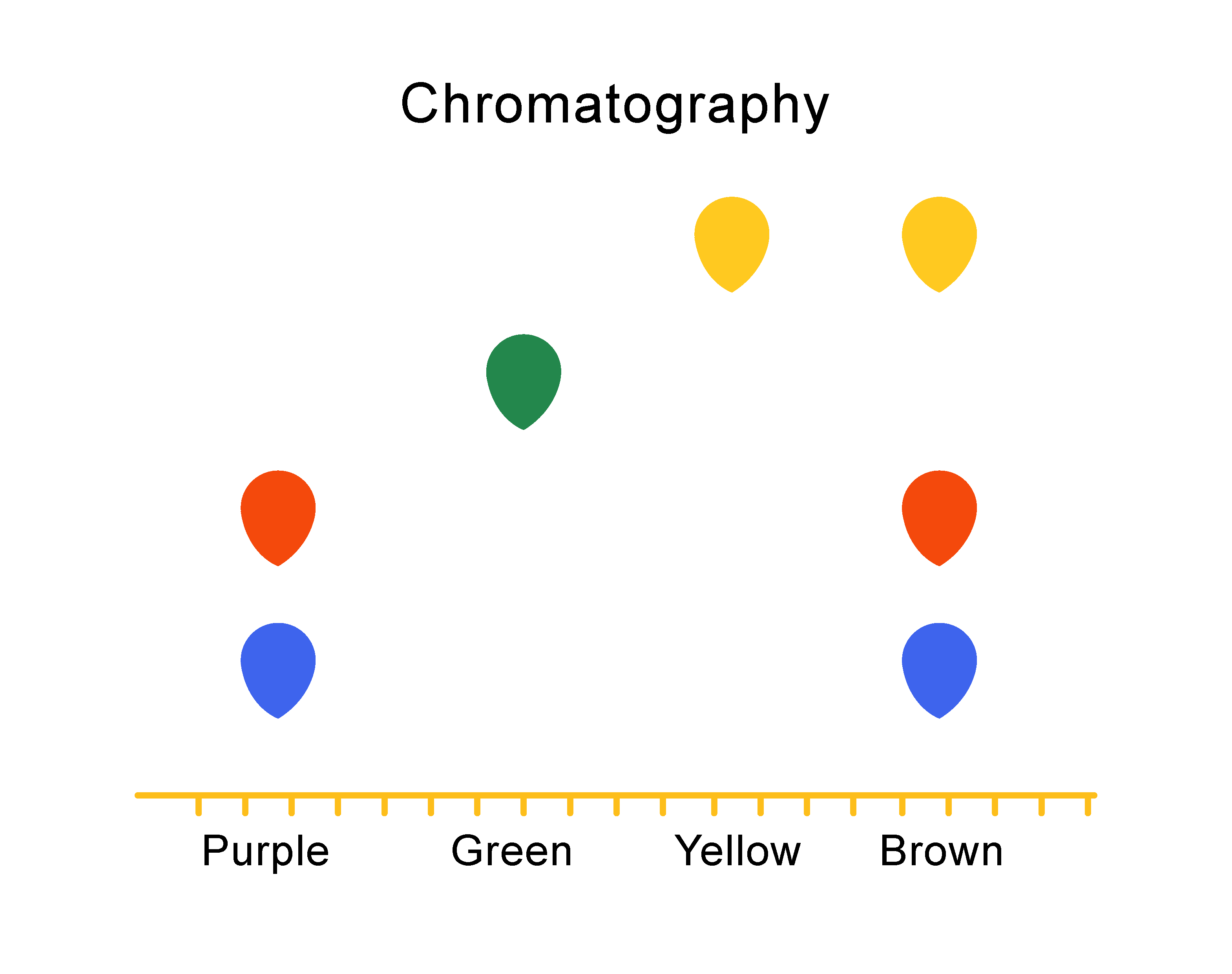
Chromatography is a technique that is used to separate different dissolved substances from one another.
The sample liquids to be tested are dropped onto special paper. The bottom of the paper is dipped in a suitable solvent (this can be water or another liquid). The different-sized particles that make up the samples travel through the paper at different speeds, so they separate at different distances.
The picture below shows the chromatography results for four different inks.
Let's try some questions on separation techniques.








