In science, there are lots of different key words that describe various things or processes. It's important to know what the different key words mean, as it will help you to feel confident when answering questions about them.
So, do you know the difference between atoms, elements, mixtures, compounds and molecules? Let's take a look at them all below to remind ourselves..........
There are 118 different elements, all found on the periodic table. An element is made from just one type of atom. An atom is the smallest particle of an element that can exist, and they're drawn as small coloured circles in diagrams.
Some elements exist on their own as individual atoms, like argon. But others go round in pairs like oxygen - these are called molecules.
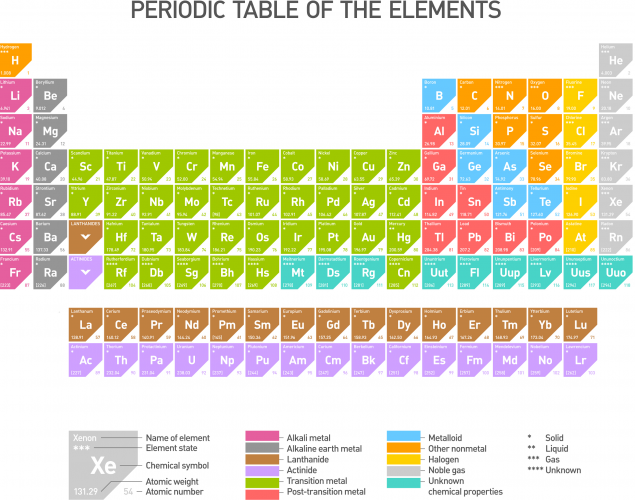
On the periodic table, the elements are arranged in a specific way, with similar elements grouped together. That's because a compound is a substance made up of two or more different elements chemically bonded together. Compounds are made up of different types of atoms. Some compounds can be made of just three atoms from two different elements like water (H2O) or lots of atoms from lots of different elements, like ethanol (CH3CH2OH) In order for a compound to be separated into its individual atoms, a chemical reaction must take place.
A compound is different to a mixture however. A mixture consists of two or more substances, but they are not chemically bonded together. So separating a mixture can be done easily.
One final thing to mention, we know that we can have molecules of elements like hydrogen and nitrogen (the diatomic elements that go round in pairs) but the term molecules can also apply to compounds. All compounds are molecules too.

That's a lot of information! Fancy trying some questions about these key words? Let's go.....








