A mixture can be easily separated by various separation techniques such as chromatography, filtration, evaporation, distillation or fractional distillation. Let's take a look at two of these techniques.
Sea water is a mixture - if we took a sample of it, we would find that it contained water, salt, sand and large bits of seaweed.

It would be easy to separate the salty sea water from the sand and seaweed by a method called filtration. That's because the sand and sea weed are solids.
The diagram illustrates how filtration is done in a scientific laboratory.
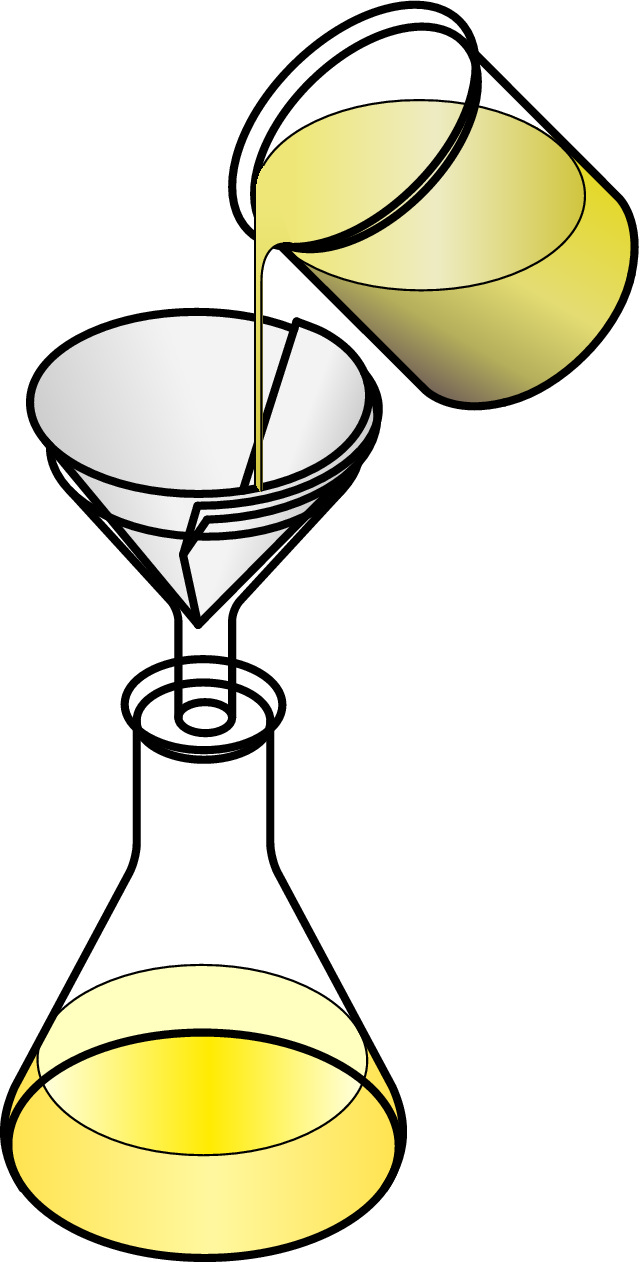
Filter paper is placed in a funnel, which, in turn, is placed in a conical flask. The mixture is poured into the filter paper. The solid material trapped in the paper is called the residue. The liquid that runs through the filter paper is called the filtrate.
So in the case of the sample of sea water, the salty water would be separated from the sand and seaweed because sand particles and seaweed are too big to go through the filter paper.
Salt particles, on the other hand, do not get trapped in the filter paper. That's because the salt is dissolved in water and the two of them make a solution. Substances that make solutions cannot be separated by filtration.
How could we separate the solution then?
We could use evaporation. During evaporation, the water evaporates away (meaning it boils and turns into a gas) leaving solid salt crystals behind. The solution can be placed in an evaporating dish and can be heated so it can evaporate faster, as in the image below.
.jpg)
Are you ready to have a go at some questions on filtration and evaporation?








