In chemistry, we refer to a pure substance as being a substance made from only one element or one compound. And nothing else.
The opposite of a pure substance is an impure substance. An impure substance is one made from more than one element or one compound, meaning it is a mixture.
Pure substances and impure substances can be identified using a simple separating technique known as chromotography.
Chromatography is a technique that is used to separate soluble substances. These are substances that are dissolved in a solvent. For example, chromatography can be used to separate the multiple different dyes in a drop of ink, some food colouring, dyes or plant pigments.
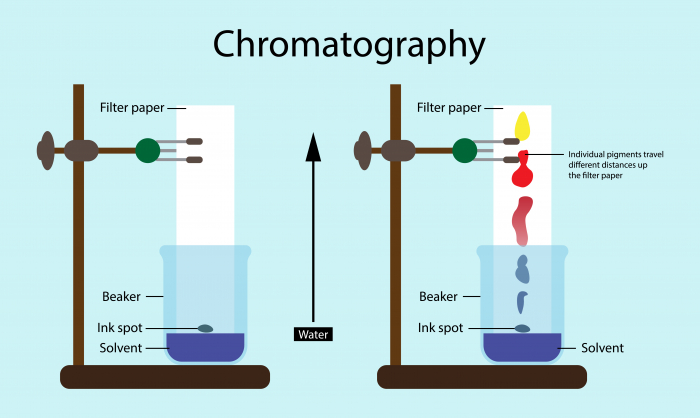
The picture below shows the chromatography results for four different inks. It's called a chromatogram. The colours they separated into can be seen by looking at the coloured dots that appear directly above the names of the different inks.
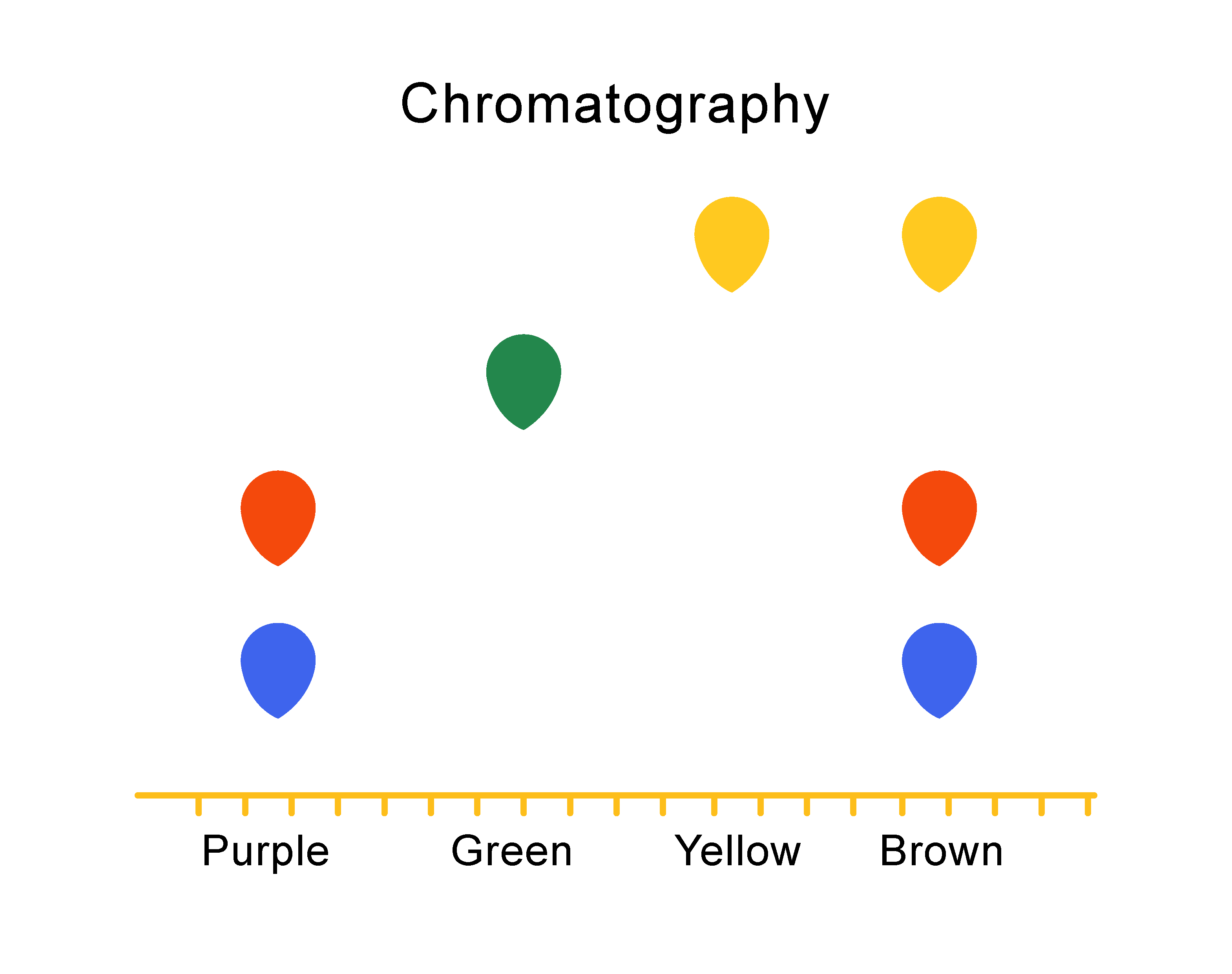
An impure substance, or a mixture, will separate into more than one spot, in a vertical column. So you can see that the brown ink is actually made up of three colours - blue, yellow and red!
On a chromatogram, one spot means that the substance is pure.
Think you can work out whether a substance is pure or impure by looking at chromatograms?
Let's try some questions.







