Matter is either classed as pure or impure.
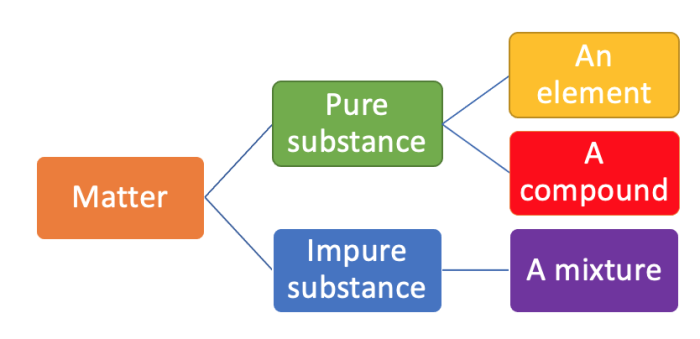
Pure substances are made up of only one type of element or one type of compound.
As soon as there is even one molecule of a different element or compound in a pure substance, it turns it into an impure substance, or a mixture.
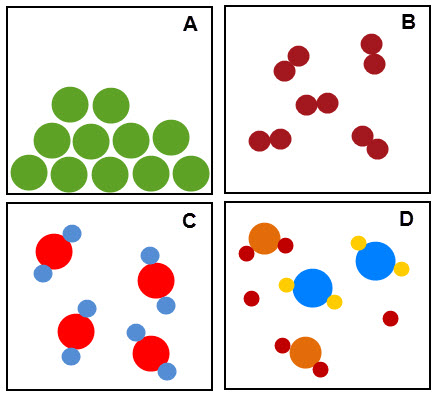
Box C shows a pure substance. It is made up of just one type of compound.
Box D shows an impure substance. It is made up of a few different elements and compounds - so it's a mixture.
Can you work out what the other boxes show?
Composition of pure and impure substances
The composition of a pure substance is pretty uniform throughout - so if you took a small sample of gold from a large block, you would see that it has the same composition no matter from where you took the sample.
.png)
But if you took a sample of impure pond water, its composition might vary depending on where you took the sample. In some samples, you might see water molecules, some salt ions and a few microorganisms. If you took a sample slightly deeper down, you might see no microorganisms but some molecules of sand with the water and salt ions.

Separating pure and impure substances
Pure substances cannot be separated into anything simpler (unless it undergoes a chemical reaction). Physical changes will not alter the chemical composition of a pure substance - for example a glass of pure water would still be pure once it is frozen or if it evaporated.
Impure substances consist of different kinds of substances (they can be elements or compounds) physically combined, but not chemically combined. That means that they can often be separated easily by physical separation, such as filtering.
Think you can distinguish between pure substances and mixtures?

Let's try some questions!








