One of the amazing things about the periodic table is that it shows you patterns in the elements – for example, all the Group 1 metals react similarly when exposed to water. There are some very good reasons for these reactions which is to do with how the electrons line up in the atoms. Here, we will be looking at the elements in Group 1 in the periodic table and some of the fascinating properties that they have.
Group 1 elements in the periodic table are called the alkali metals. They are lithium, sodium, potassium, caesium and rubidium. Can you find them on a periodic table? They are called Group 1 elements because they all have just one electron in their outer shell. Take a look at their electron structure below:
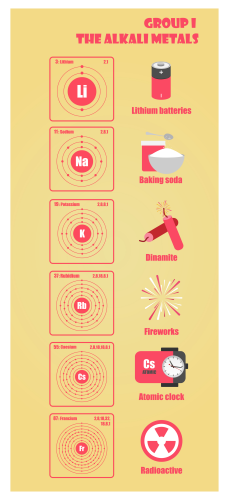
They all react vigorously with air and water, so they must be stored in oil to stop air or water getting to them – it could cause an explosion.
Sodium is more reactive than lithium, and potassium is more reactive than sodium. You can tell this because potassium reacts extremely vigorously and quickly with water, whereas lithium reacts less quickly and vigorously. As for caesium and rubidium - their reaction is.... explosive! So, you might have noticed by now that as you go down the periodic table, you are increasing the reactiveness of the elements.
“But why are they called the alkali metals?” I hear you cry from the rooftops. Well, fear not, the answer is here! Here is the general chemical equation - what do you notice about the right-hand side?
metal + water  metal hydroxide + hydrogen
metal hydroxide + hydrogen
Well, any time something is called a hydroxide, it is an alkali – so when the Group 1 metals react with water, they form an alkali and hydrogen. Hence the term alkali metal.
So, if we were reacting lithium with water, it would look like this
lithium + water  lithium hydroxide + hydrogen.
lithium hydroxide + hydrogen.
And if we were to use potassium instead, it would look like this:
potassium + water  potassium hydroxide + hydrogen.
potassium hydroxide + hydrogen.
Get the picture?
There are other patterns that you need to remember as well – take a look at them:
| Metal | Softness | Colour | Reaction with oxygen | Reaction with chlorine |
| Lithium (Li) | Hard to cut with a knife. | Grey colour, silver when cut. | Makes Li2O (lithium oxide) | Makes LiCl (lithium chloride) |
| Sodium (Na) | Softer, easy to cut with a knife. | White colour, silver when cut. | Makes Na2O (sodium oxide) | Makes NaCl (sodium chloride) |
| Potassium (K) | Very soft - can be squeezed in tweezers. | Grey, silver when cut. | Makes K2O (potassium oxide) | Makes KCl (potassium chloride) |
Study the table above very carefully because you will need to know how the properties of these elements change. Are you ready to have a go at some questions now?








