The main elements found in living organisms are carbon (C), nitrogen (N), oxygen (O2) and hydrogen (H2). These elements can combine in different ways to produce a variety of different compounds, all with a different use.
As we know, plants make their own food through a process called photosynthesis in which they use the sunlight to change water (H2O) and carbon dioxide (CO2) into glucose and oxygen.
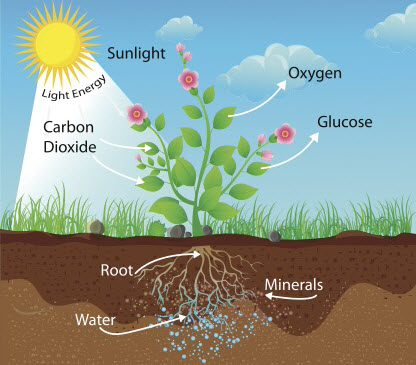
H2O and CO2, however, only contain hydrogen, oxygen and carbon which means that plants must get the nitrogen and other elements that they need from another source. The source of these nutrients is the soil.
Nitrogen, phosphorous and potassium (NPK) are the essential elements for healthy plant growth. As shown in the diagram above, these nutrients are found in the soil and are taken in through the roots of plants as dissolved compounds.
Have a look at why these minerals are vital to healthy plant growth (and therefore, to us!):
| Essential element | Use | Sign when plant is lacking this element |
| Nitrogen (N) | Making protein and DNA | Smaller than normal leaves and shoots and brown leaves |
| Phosphorus (P) | Making important plant chemicals | Yellow spots on leaves |
| Potassium (K) | Needed for chemical reactions | Poor growth and purple leaves |
The Nutrient Problem

Once a plant dies and begins to decompose, all of the absorbed nutrients are then returned to the soil for other plants to use. This is known as the nutrient cycle.
The problems occur, however, when it comes to harvesting crops. Many plants are grown by farmers to be used and sold. When the plants are removed from the soil, so are the nutrients, causing problems for future crops planted in the same soil.
The Solution
Farmers often use chemicals called fertilisers to provide the soil with the essential elements needed for plant growth.
Natural fertilisers such as manure and compost (rotten plant matter) are often used as they have no negative effects on the environment.
The increasing demand for food, however, has resulted in the use of artificial fertilisers. These are made by the chemical industry and contain ammonium, nitrate, phosphate and potassium compounds. To be effective, a fertiliser must be soluble in water so it can absorbed by the plant roots.
The Effect of Fertilisers on the Environment
The use of artificial fertilisers can have many negative effects on the environment. Fertilisers often dissolve in the rainwater and get washed away into rivers and streams, which can lead to several problems including the depletion of several marine species and the contamination of drinking water.
Once you are happy with all this information, carry on into the activity itself.







