So we know that catalysts speed up the rate of a reaction, but how do they do this?
Well, for a chemical reaction to happen, the reactants need to collide with each other with enough energy to break the bonds between their atoms. This then allows the atoms to rearrange and form the products.
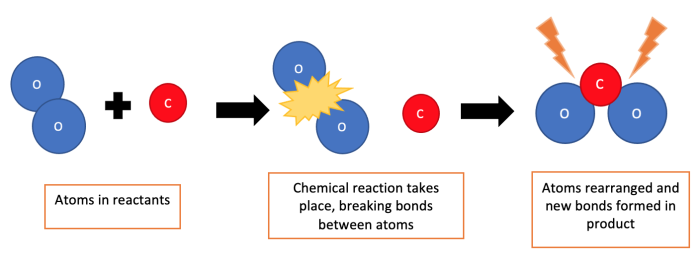
Activation energy is the minimum energy needed by the reactants for these ‘successful collisions’ to occur. It's the initial energy needed to get a reaction going. It is the energy barrier that must be overcome for reactants to be converted to products.
The presence of a catalyst provides an alternative reaction pathway which has a lower activation energy. In other words, a catalyst lowers the activation energy for a reaction. This increases the frequency of successful collisions as more of the particles will have the energy to allow the collisions to occur.
The higher the activation energy, the slower the chemical reaction. A low activation energy means that more molecules will have the required energy, and the reaction will occur faster. So a catalyst increases the rate of reaction by lowering the activation energy.
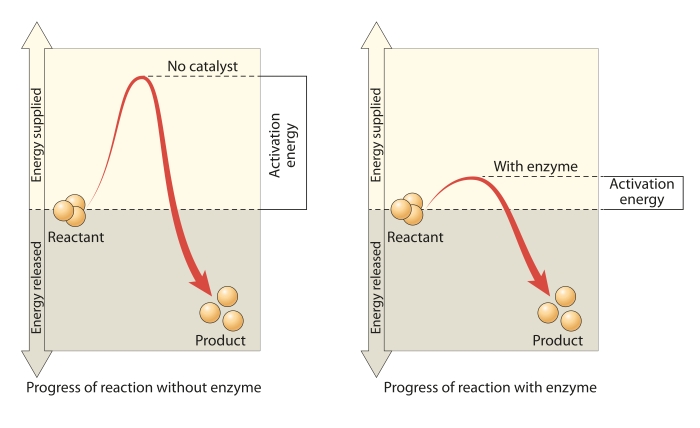
As you can see in the picture, the activation energy required for a reaction without a catalyst is quite high. But by adding a catalyst, or enzyme, it lowers the activation energy required for the reactants to turn into products.
Let's try a few questions on this now.







