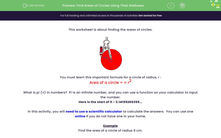
Most gases look very similar to each other. If we have some gas (maybe made in an experiment), how can we tell what the gas is?
Gas tests are four short experiments which allow us to decide whether or not an unknown gas is the gas we think it might be. There are tests for hydrogen, carbon dioxide, oxygen and chlorine. You need to know the experiments and what the results mean - so here they are!
The hydrogen test is called the 'squeaky pop' test.
If we have some gas, and put a flame near it, the hydrogen reacts with oxygen in the air, and we hear a distinctive squeaky pop. If we don't hear the popping sound, there is no hydrogen present.
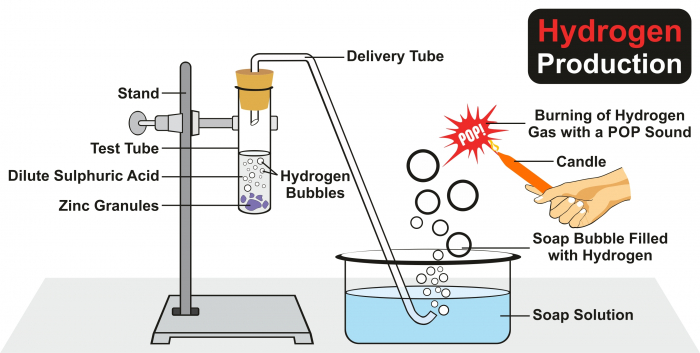
In the experiment above, acid + metal → salt + hydrogen, and the hydrogen collects in the soap bubbles. If we put a flame near the bubbles, we will hear a squeaky pop. When the flame is in contact with the hydrogen, the reaction which happens is hydrogen + oxygen → water.
The carbon dioxide test is called the limewater test. If we bubble carbon dioxide gas through limewater, the limewater becomes cloudy and white. If this doesn't happen, there is no carbon dioxide present.

Limewater is calcium hydroxide dissolved in water - it's a clear, colourless solution. When it reacts with carbon dioxide, it makes calcium carbonate. Calcium carbonate is not soluble in water, so we see white clouds. The reaction is calcium hydroxide + carbon dioxide → calcium carbonate + water.
The oxygen test uses a glowing (but not properly lit) splint. If we place this in oxygen, it will relight, so that it burns properly.
The chlorine test requires a piece of damp blue litmus paper. If chlorine is present, the litmus paper will first turn red, and then it will bleach white.
And that's it! The best way to use these tests is to predict what you expect to happen, by thinking about the reaction equations before doing an experiment. Then you can decide which tests are worth doing to check that the gas made is the one you expect, and that the reaction is doing what you think it should.
Now for some questions!