Chemicals react together when their particles collide with each other - to collide means to bump into each other or to hit each other. Some reactions are very slow and others very fast but there are five factors that affect the rate of a reaction:
Concentration
Temperature
Surface Area
Pressure
Catalysts
Collision theory
The collision theory states that the more collisions between particles there are, the faster the reaction. The particles must be moving very fast and have lots of kinetic energy for collisions to occur.
'Kinetic' means 'moving', so this type of energy is about the movement and collision of particles.
Let's see how the five factors above affect the number of collisions between particles.
Concentration
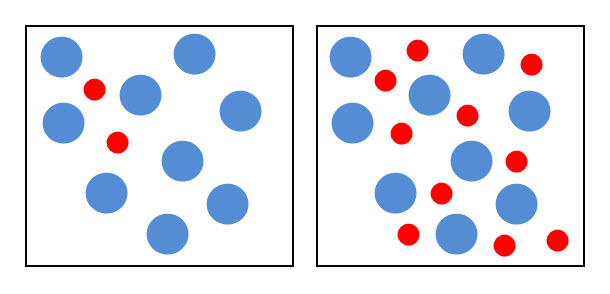
If the concentration of one or more of the reactants increases, the particles become more crowded. The diagram below shows the particles of two chemicals. The box on the right contains more red particles, representing one of the chemicals about to react. This means that the concentration of the 'red' chemical is now higher but the particles are still in the same space. This increases the probability of collisions and the rate of the reaction.
Temperature
When the temperature increases, the particles have more energy, as an increase in temperature means more thermal energy (heat) is present. More heat energy gives the particles more kinetic energy. More kinetic energy means more movement, so the probability of collisions increases and so does the rate of the reaction.
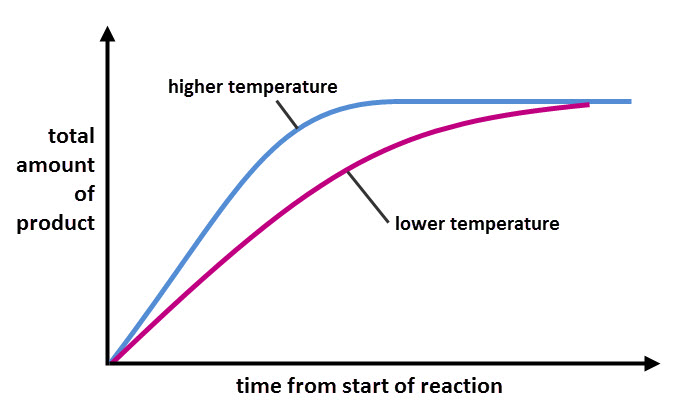
The graph below shows the rate of the same reaction in different temperatures. Note that the blue line, which shows the higher temperature, is much steeper than the purple line.
Surface area
When a solid chemical is broken down into smaller pieces - or even a powder - there are more particles that can react, as the diagram below shows. We say that a chemical in a powder form has a greater surface area than the same mass of the same chemical in a solid block form. An increased surface area allows for more collisions and the rate of the reaction increases.
.jpg) |
.jpg) |
Pressure (in gases)
An increase in pressure speeds up a reaction. It has the same effect as an increase in concentration. The way you increase pressure on a gas is by squeezing it into a smaller volume but the mass remains the same. This results in the same number of particles moving about in a smaller volume, which increases the number of collisions and the rate of the reaction.
Catalysts
A catalyst is a chemical that speeds up a reaction without being used in it. Catalysts are specific to reactions, so the catalyst for one reaction would not work for another.
Now it's time for some questions to see how much you can remember.








