A pure substance is a substance made from only one element or one compound.
The opposite is an impure substance.
An impure substance is one made from more than one element or compound, meaning it is a mixture.
Here is a test tube containing an unknown liquid. How could we test to see if the liquid is pure or impure?

We can use a simple separating technique known as chromotography.
Chromatography is a technique that is used to separate soluble substances. These are substances that are dissolved in a solvent. For example, chromatography can be used to separate the multiple different dyes in a drop of impure ink, some food colouring, dyes or plant pigments, or show that the sample is pure.
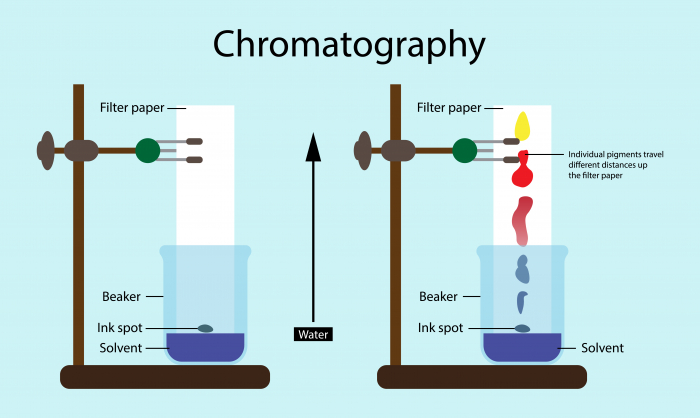
If we put a single drop of the unknown liquid on a pencil line drawn on a blank chromatogram, we can use chromatography to see if it is pure or impure.
.jpg)
Once complete, you can look at the chromatogram and see the results

As you can see, the liquid has been separated into three different spots in a vertical column, which have all travelled up the paper at different rates. This means that the liquid we tested is impure. It must have been a mixture of three different compounds or elements, which is why there were three spots produced on the chromatogram. A pure substance would only produce one spot, showing it is made of only one type of element or compound.
Think you can work out whether a substance is pure or impure by looking at chromatograms?
Let's try some questions.








