How do gas particles behave?
Gas particles move with fast, random motion and have large spaces between them. They have weak forces of attraction between them, which allows the particles to move around freely.
The greater the temperature of a gas, the faster the particles will move.
What is gas pressure?
Gas pressure occurs when particles collide. This may either be particles colliding with each other, or colliding with the walls of their container. These collisions exert a force, and it is the force that causes pressure. Gas pressure is measured in Pascals.
How do you increase gas pressure?
The more collisions that occur every second, the greater the pressure of the gas. So, in order to create more pressure, you can increase the temperature of the gas, reduce its volume (which is called compression), or simply add more gas particles into a container. All of these factors will cause more frequent collisions, and therefore increase the pressure.
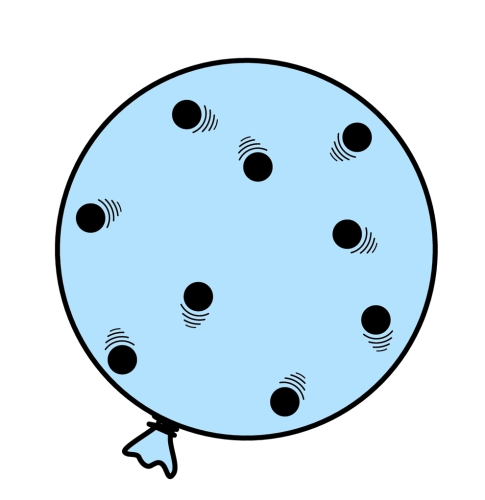
For example, in this balloon, the air particles inside are flying around randomly, and colliding with each other (as well as the walls of the balloon). If you heated the balloon, shrank it, or pumped more air into it, then it would experience more pressure. The pressure acts outwards from the centre (as the pressure inside is much greater than the surrounding air) and too much pressure would even cause it to burst.
Happy with all that?

Let's get started on the questions then.







