Fractional distillation can be used to separate a mixture of liquids. This is possible because the different liquids have different boiling points.
Boiling point is the temperature at which a liquid turns into a gas. For example, the boiling point of water is 100oC, whereas the boiling point of ethanol is 78oC. So if you had a mixture of ethanol and water and wanted to separate them, you could heat the mixture up and would find that the ethanol would evaporate first. By catching the ethanol vapour and directing it through a condenser to cool it back down to a liquid, you would end up with liquid ethanol and liquid water, separated!
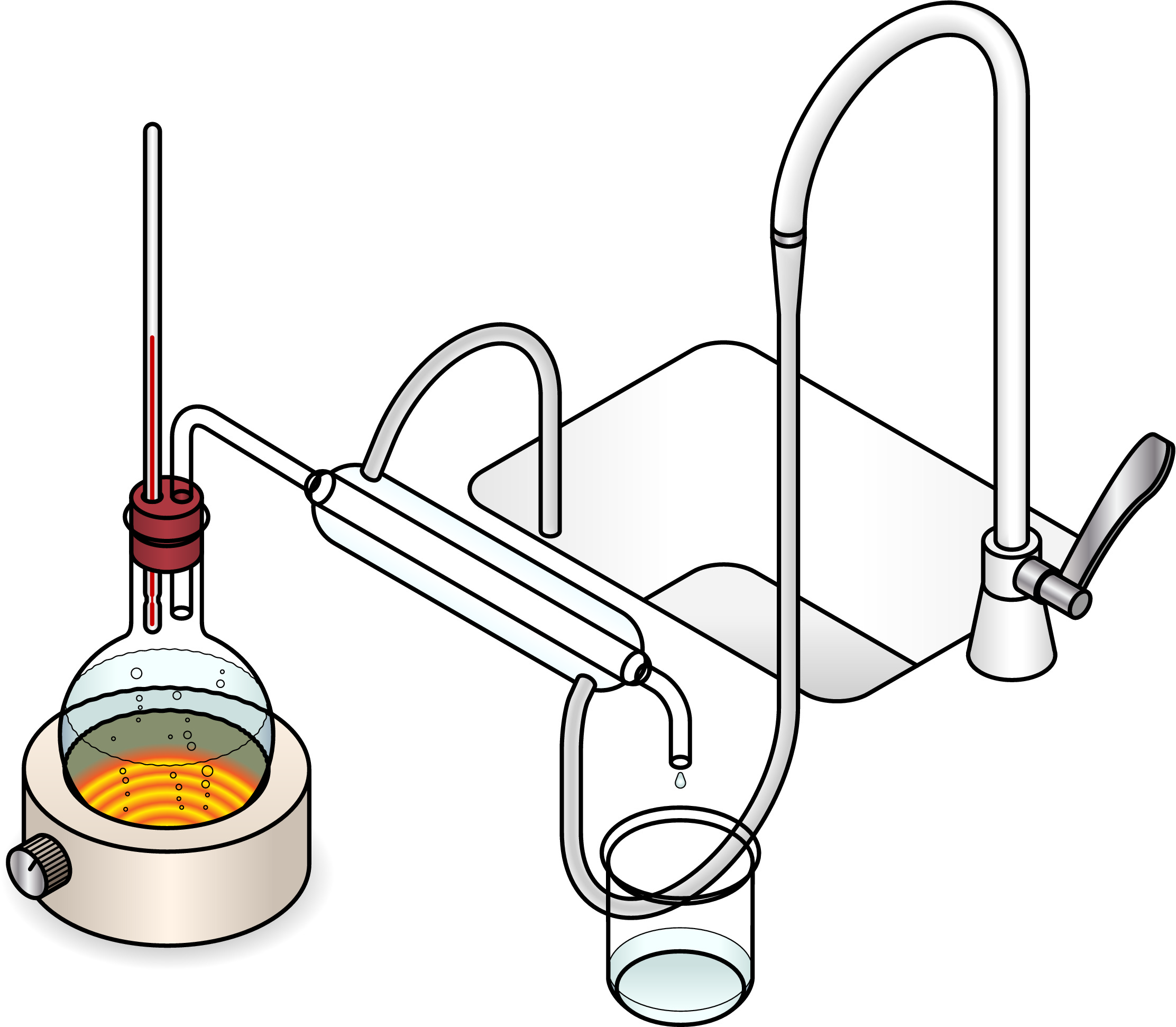
Fractional distillation can also be used to separate crude oil into the many compounds that it is made up of.
Crude oil is a mixture. It is found deep under ground. It is not very useful as it is, but some of the liquids that are within the mixture are very useful compounds, such as petrol and diesel.
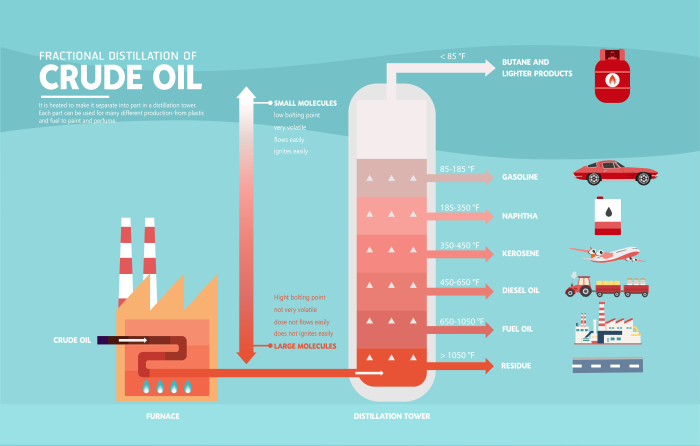
Steps of fractional distillation of crude oil:
Crude oil is heated to very high temperatures in a furnace, until it turns into a gas or vapours.
It is then pumped into a distillation column.
The column is hot at the bottom and cooler at the top.
The vapours begin to rise up the column.
The vapours will condense at different parts of the column - at a temperature just below their boiling point.
Compounds with the highest boiling points will condense first, like residue.
Once the vapours have condensed the liquid runs off from the tower.
The vapours will continue to rise higher and higher, with different compounds condensing at different stages.
Compounds with the lowest boiling points will be the last to condense, at the very top of the tower, like butane gas.
We can see that from crude oil there are lots of different, useful fractions produced. This is a reason why fractional distillation is so great!
Shall we try the questions on separating mixtures of liquids?









