For thousands of years, people have wondered what things are made of. In the time of Ancient Greece, people had the idea of atoms - tiny blocks that were so small that they couldn't be broken into smaller pieces.
We now know that there are about 120 different types of atom and that everything around us is made of different atoms joined together in different patterns. Atoms are a little like toy blocks. You can use a few types of block to make all sorts of things, depending on how you join them together.

Each atom has a name and a symbol. You need to know the names and symbols for the first twenty. This looks scary, but if you test yourself on a few at a time, you will soon know them all by heart.
| Hydrogen | H | Carbon | C | Sodium | Na | Sulfur | S |
| Helium | He | Nitrogen | N | Magnesium | Mg | Chlorine | Cl |
| Lithium | Li | Oxygen | O | Aluminium | Al | Argon | Ar |
| Beryllium | Be | Fluorine | F | Silicon | Si | Potassium | K |
| Boron | B | Neon | Ne | Phosphorus | P | Calcium | Ca |
The symbols are always either a capital letter or a capital letter followed by a lower case letter. Most of the symbols start with the same letter as the atom's name, but not all - can you see which atoms have symbols which don't match their name?
Because atoms are so small, we need to think about how to combine them to make things. There are three main ways of doing this:
In an element, all the atoms are the same type. In pure gold bars, all the atoms are gold atoms. When a scientist draws a picture of the atoms in a gold bar, all the atoms look the same:

.png)
In a compound, different atoms are joined together (chemists say 'bonded'), but in fixed patterns. Water is a compound of hydrogen and oxygen, with two hydrogen atoms for each oxygen atom. In the atomic picture, the two white balls are hydrogen atoms and the red ball is an oxygen atom:

.png)
Table salt is a compound of sodium and chlorine, with one sodium atom for each chlorine atom. In this atomic picture, purple means sodium and green means chlorine:
.jpg)
.png)
In a mixture, different elements or compounds are in the same container, but they are not bonded, and they have no pattern. Seawater contains water and dissolved salt, but they don't make a new compound - they are still water and salt.
If you have a mixture, you can usually separate it back into different pure elements and compounds. Some ways of separating a mixture are:
Filtration, using a sieve or filter paper. Liquids go through the filter, but solids get trapped.

Crystallisation, where we remove the liquid by heating it so that it evaporates - the solid gets left behind.
Simple distillation, where we heat a liquid so that it evaporates. We collect the vapour and cool it back down to collect a pure liquid.
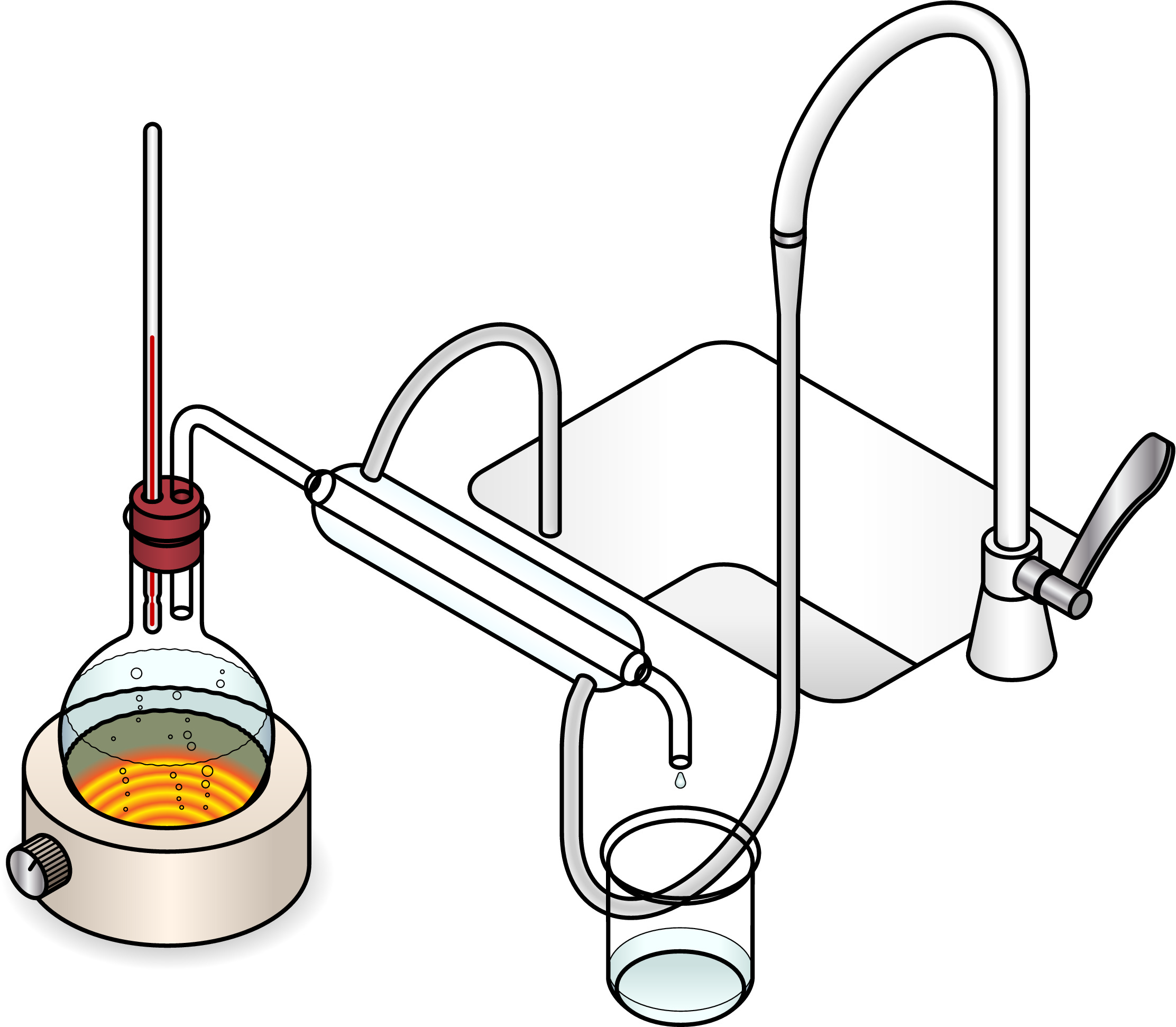
Fractional distillation, which is like simple distillation except that we carefully collect different liquids which condense at different temperatures.
Chromatography separates different substances dissolved in a solution, for example, different colours mixed together to make ink.
You will learn a lot more about these different methods in another activity. For now, the important thing to remember is that mixtures are easy to separate into pure elements or pure compounds. Converting a pure compound into separate pure elements is much harder because it means breaking chemical bonds.
Now let's move on to some questions.








