Ionic bonding works brilliantly when a compound has a metal and a non-metal in it. The metal atom loses unwanted electrons, the non-metal takes electrons it does want, and everything works well.
What if there isn't a metal and a non-metal? We need other types of bonding - covalent bonding happens between two non-metal atoms.
It's easy to mix up ionic and covalent bonding, so watch out for the ways in which they are similar and different.
A lot of very important substances are covalently bonded, so make sure you can remember the names and formulas of these ones:
| Name | Formula |
| Water | H2O |
| Ammonia | NH3 |
| Methane | CH4 |
| Carbon dioxide | CO2 |
If both atoms are non-metals, there isn't a source of spare electrons to complete electron shells, so ionic bonding can't happen.
For example, imagine having two fluorine atoms. Each atom has seven out of eight positions filled in its outermost shell, and neither atom is going to give up any of its electrons. To solve this problem, the two atoms share one electron each.
The shared electrons sit in parts of the shell between the two atoms. A tiny fairy sitting in the middle of each atom would see a complete outer shell, but this only works if the two atoms are exactly the right distance between each other, making a bond between the atoms. This type of bond is called a covalent bond, and we can draw a dot-and-cross diagram for this sort of bonding as well. The covalent bond is the part where the circles touch or overlap.
Each of the fluorine atoms has put one of its electrons into the shared part of the shell, so this is called a single covalent bond, which can be written as F-F.

You can do the same thing with compounds.
Carbon dioxide is carbon and oxygen, and they are both non-metals. Carbon needs another four electrons, and oxygen needs another two. To make carbon dioxide, each bond between carbon and oxygen needs two electrons from the carbon and two from the oxygen. If you look round the oxygen atoms, they seem to have eight electrons, and if you look round the carbon, it also seems to have eight electrons. The carbon and oxygen each put two electrons into the shared part of the shell, so this is a double covalent bond, which we can write as C=O.
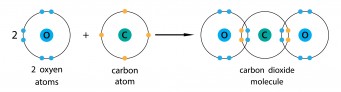
In this diagram, the electrons from the different atoms are shown in differerent colours. Another way of showing this is to use different symbols - for example dots and crosses.
Be really careful not to mix up the different dot-and-cross diagrams.
Remember:
If it's a metal with a non-metal, you have ionic bonding. One or more electrons move to the non-metal, but the atoms stay separate.
If it's a non-metal with a non-metal, you have covalent bonding. Some electrons are shared, so the atoms are drawn touching or overlapping.
Polymers are an important group of materials with covalent bonding. They are the basis for plastics, and many parts of the human body. They are very long, with a chain of carbon atoms down the middle. This picture shows polyethene, which is the plastic used for shopping bags.
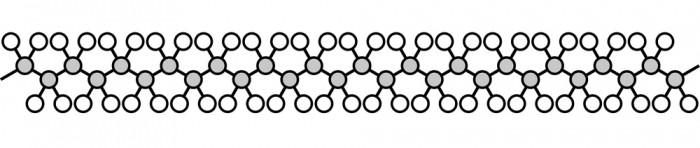
It's not worth drawing out all the atoms, and often we don't need to draw them with the bonds at their exact angles. Instead, scientists simplify the diagram like this:
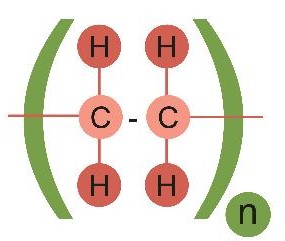
This makes it easier to see which atoms are bonded to others. The repeating part of the structure is put in brackets, and the n tells us that there are many repeated copies of that making the complete polymer. You'll learn a lot more about polymers in another activity.
We've seen several different ways of drawing these structures. If you draw the atoms in their true positions, the picture is more realistic, but it can be hard to see enough of the structure to understand how the different atoms link together. If we simplify the diagram, it is easier to understand, but the disadvantage is that we lose some of the information about the structure.
Now it's time for some questions.







