Will diffusion happen in the same way in solids, liquids and gases?
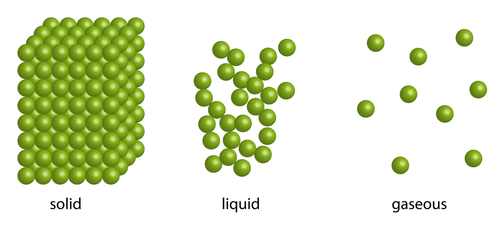
In a solid, the tiny particles that make up the solid are very close together in a neat regular arrangement, all touching each other and moving only by vibrating on the spot. They cannot move much at all. So diffusion can't really happen in solids.
The particles in a liquid are arranged in a more random way. They are close together, most of them touching each other, but there are some small gaps. So the particles can move around a little and over each other. So diffusion can take place in liquids, like when tea particles diffuse out of the tea bag into a cup of boiled water.
The particles in gases are much freer, and move around much more and faster than in liquids. The particles in a gas are spread out much further than in liquids or solids. Diffusion happens really quickly in gases as gas particles are spaced far apart and also because they bump into each other more often, sending particles of substances flying in all directions, helping them spread out! This is why smells spread throughout a room really fast.
Does that all make sense?

Let's have a go at some questions on this now.








