What is internal energy?
All objects, at any temperature, have a property known as internal energy. If you added up the total amount of energy of every single particle inside a substance, that would be its internal energy.
As substances contain billions of particles, we can't analyse every single particle at a given moment. However, we can easily compare two substances to see which one has more internal energy, as it is directly proportional to temperature and mass.
If an object has a greater temperature, its particles will move faster (and therefore they will have more kinetic energy). This is directly proportional to the object's internal energy. For example, an object at 2ºC has double the temperature of an object at 1ºC, and its particles have twice as much energy.
The other factor, mass, depends on the number of particles. So, if there are more particles inside a substance, it will have more internal energy. For instance, an object with a mass of 2 grams has twice as much internal energy as an object (at the same temperature) with a mass of 1 gram, as it contains twice as many particles.


If you had a swimming pool at 10ºC and a fish tank at 20ºC, the swimming pool's water particles would have half the energy of those in the fish tank, but the mass of the water in the swimming pool will be many, many times greater than the fish tank. As internal energy is proportional to both temperature and mass, the swimming pool has more internal energy.
Is this the same as heat energy?
We are referring to the temperatures of objects, but internal energy is not the same as heat energy.
Internal energy is just a property of a substance, telling us how much energy it is storing. It is important to understand that internal energy does not leave the object. Heat energy, on the other hand, is the transfer of energy from one object to another. It occurs when a hot object and a cold object come into contact with each other. So, for this reason, they are different forms of energy.

A cup of coffee has internal energy due to its temperature and its mass, but if it is transferring this energy to the surroundings, that is an example of heat energy.
Temperature-time graphs
Finally, we will look at temperature-time graphs, as these are useful in understanding how internal energy can change as something is heated.
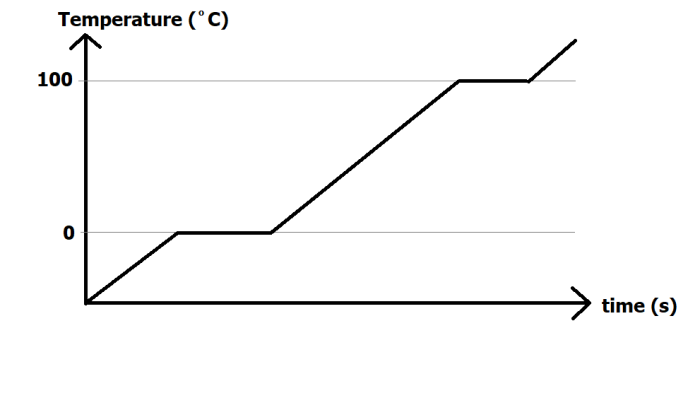
In the graph above, an ice cube is continuously heated until it melts, turns to water, gets even hotter, and then evaporates into water vapour.
Any regions of the graph where the temperature is rising means an increase in internal energy. (The two quantities are directly related).
You will notice that there are flat regions on the graph. For water, these occur at 0ºC and 100ºC. The reason these parts appear on the graph are due to changes of state. When an ice cube is melting or when water is boiling, the heat energy that is being supplied to the substance is used for breaking particles apart. It cannot be used to make them move any faster, and for this reason, the temperature does not change when there is a state change.
If the heater was more powerful, the diagonal lines on the graph would become steeper (as the temperature would rise in a smaller amount of time), but no matter how powerfully you heat the substance, there must always be flat regions when the substance changes state. The line could never be a single diagonal line from solid to gas.
Right, let's have a go at some questions on this now.
.jpg)







