Group 0, or the noble gases
The noble gases are found on the far right-hand side of the periodic table. This group is called Group 0.
It's neat that they are in Group 0 because the word 'nothing' is a good description of the behaviour of these elements! The noble gases - helium, neon, argon, krypton, xenon and radon - are all very unreactive. This means that they are very hard for chemists to detect. For example, the list of elements that Newlands used for his octaves model went straight from hydrogen to lithium, missing out helium, and from fluorine to sodium, missing out neon.
The main way that noble gases are used is to make an inert atmosphere. 'Inert' means that it does not undergo chemical reactions. For example, light bulbs are filled with argon. This is because the thin wire in the middle of a light bulb gets very hot when the light is switched on and would burn away if there were oxygen inside the light bulb.
The reason that noble gases are unreactive is that they all have a stable outermost electron shell. Here's the electron structure of argon:

Helium has 2 electrons, filling the first shell. Neon is 2.8. Argon is 2.8.8. All the noble gas elements have complete outermost shells, so they do not need to gain or lose electrons. As a result, they do not make molecules - we find them as single atoms instead. The other name for this is monatomic. They also (with very few exceptions) do not undergo chemical reactions.
As you go down Group 0, the gases get denser. That makes sense because the individual atoms have more particles and so more mass.
Also, as you go down Group 0, the boiling point increases, but is always very low. It makes sense that Group 0 elements have very low boiling points - the forces between the atoms are very weak, so it doesn't take much energy to break the atoms apart. Elements that are lower in the periodic table have heavier atoms, so it needs a bit more energy to make the atoms move freely.
Metals vs non-metals
The properties of different groups of the periodic table depend a lot on the electrons - that's why it's so important to think about where the electrons are in different atoms and ions. Look at the whole periodic table again:
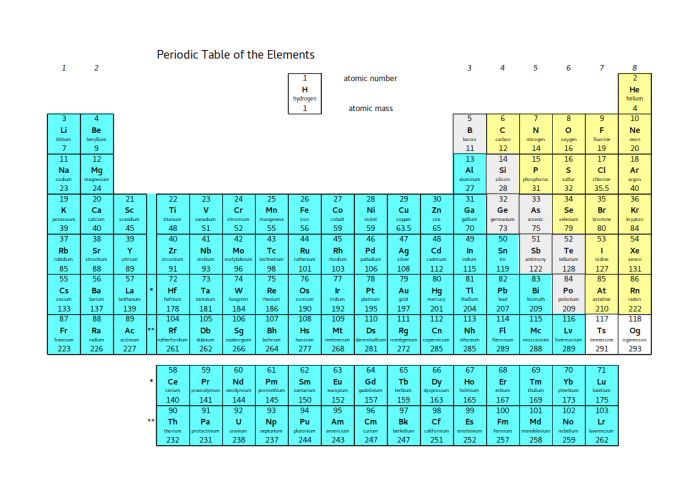
The elements shaded blue all have the properties of metals - they are shiny, they conduct heat and electricity and they are ductile. In terms of electrons, they all become stable by losing electrons.
The elements shaded yellow all have the properties of non-metals - their solids are dull, brittle and poor conductors, and many of them are liquids or gases at room temperature. Except for the noble gases, they all become stable by gaining electrons.
In between the two coloured blocks, there is a diagonal line shaded grey. These elements are called metalloids. Depending on the exact situation, they can either gain or lose electrons. This means that they can either behave like metals or non-metals. This property means that metalloids are very useful - electronic circuits are made of silicon because it can easily be switched between metal-like and non-metal-like states.
All these ideas about the periodic table end up depending on the electron arrangements of different elements. Atoms will gain or lose electrons to achieve a stable outer shell - that's usually when there are eight electrons in the outer shell. The reason that noble gases are so unreactive is that they have a stable outer shell already.
Now it's time for some questions.








