Here is some chocolate that has been left out in the sunshine, a whistling kettle, and water dripping down a bathroom mirror.



What do these three things have in common?
Answer: They all involve a change of state.
A change of state is simply when a solid, liquid, or gas turns into a different state of matter. This will be due to a change of temperature, which changes the way the particles are arranged. It also affects the strength of the forces between molecules.
The changes of state are summarised below:
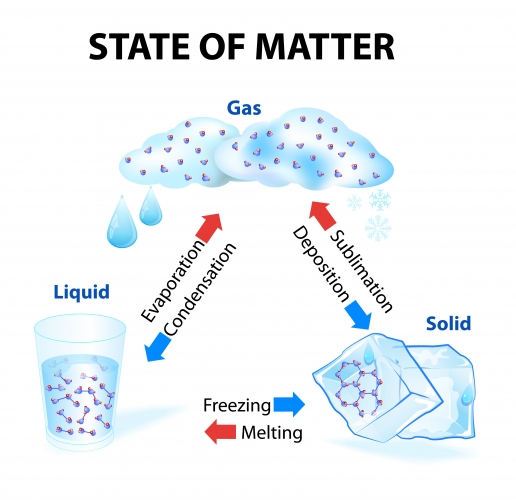
Notice that some of the arrows are red and some are blue.
The red arrows represent changes that involve an increase in temperature (i.e. melting, evaporation, and sublimation need you to heat something up). These all cause the particles to move faster and further apart, as well as weakening the forces between molecules.
The blue arrows represent changes of state that involve a decrease in temperature (i.e. condensation, freezing, and deposition need you to cool the substance down). In these situations, the particles slow down, move closer together, and the forces between molecules become stronger.
.jpg)
Let's try some questions!








