A large part of crude oil is residue and consists of very long hydrocarbon molecules. Some can be used for bitumen, to make tar for roads, but there is more residue than we really want. How can we avoid wasting it?
Cracking is the name given for a thermal decomposition process which breaks long hydrocarbon molecules into shorter ones. All that is needed is to heat long-chain hydrocarbons, so that they turn into vapour. The vapour is passed over a hot catalyst (called zeolite) to speed up the reaction. Alternatively, the hydrocarbon vapour can be mixed with very hot steam. This can be done on a very large scale in a chemical works, but it also works as a small-scale experiment:

Molecules in a cracking reaction
Let's look at the molecules in this reaction close-up:
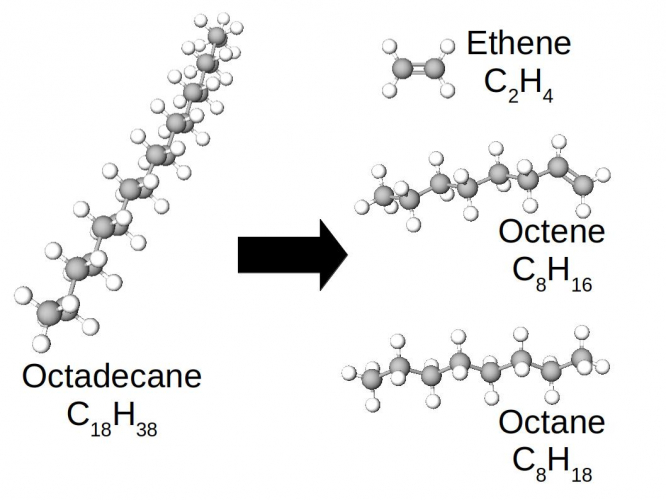
In this case, the long molecule of octadecane has been broken into three smaller pieces.
The ethene is the one which collected as a gas in the experiment - it has such weak intermolecular forces that it is a gas at room temperature.
One of the products is a smaller alkane (octane), but the other two are not. Look at the bond between the carbons in ethene, or the rightmost C=C bond in octene. They are double C=C bonds, not single C-C bonds.
When the octadecane molecule cracked into three pieces, two of the C-C bonds in the backbone were broken. In effect, these broken bonds looped back into the molecule, so that each carbon still had four covalent bonds.
Ethene and octene are examples of another series of hydrocarbons, called the alkenes.
Alkenes
Alkenes are another homologous series of hydrocarbon molecules, like alkanes. The difference is that alkenes have a double C=C bond somewhere in the structure, while in alkanes, all the bonds are single C-C bonds.
Alkenes have isomers because the double bond can be in different parts of the structure. Their general formula is CnH2n. The names follow the same pattern as for alkanes:
| Number of carbon atoms | Name | Formula |
|---|---|---|
| 2 | C2H4 | Ethene |
| 3 | C3H6 | Propene |
| 4 | C4H8 | Butene |
| 5 | C5H10 | Pentene |
Starting with pentene, the alkene names have the same prefixes used to name shapes: C6H12 is hexene ('hex' is six, as in hexagon) and so on.
Scientists also talk about these compounds as saturated and unsaturated. A saturated compound only has single C-C bonds, while an unsaturated compound has one or more double C=C bonds, or even triple C≡C bonds. The point is that an unsaturated molecule can also bond to something else (by breaking up the double or triple bond), whereas a saturated compound cannot. The C=C bond in alkene molecules is its functional group - it's the characteristic part of the molecule where a chemical bond can be made.
This gives us a chemical test to tell saturated and unsaturated compounds apart. Bromine water is normally orange. If we add a saturated compound to it, it cannot react with the bromine, so nothing happens. If we add an unsaturated compound, the bromine in the water will bond with it, where the double or triple bond was. The water will turn colourless because it will no longer have bromine dissolved in it.
This means that bromine water will stay orange if we add an alkane, but become colourless if we add an alkene.
Alkenes are useful because they are the raw material for many plastics. We can break open one of the two bonds in the double bond, and that allows us to join alkene molecules together to make materials called polymers - there is another activity to help you learn about these.
So, to summarise, cracking happens when we heat long alkane molecules into a vapour, and pass the vapour over a catalyst, or mix it with steam.
This breaks long alkane molecules (which aren't very useful) into smaller molecules. Some of these are useful small alkanes, and others are alkenes, which can be used to make plastics.
Now let's move on to some questions on this.







