In this activity, you will be introduced to the terms exothermic and endothermic to describe reactions or processes, and look at examples of each type.
Exothermic reactions
You may have already worked out that exothermic relates to thermal energy, and you would be correct. Any exothermic reaction is one that transfers heat energy to its surroundings. (Think of it as a reaction that releases heat).
If an object is transferring heat away from itself in an exothermic reaction, the object will become cooler, while it makes the surroundings warmer.
.jpg)
The most obvious example of an exothermic reaction is combustion (which is the chemical reaction that causes objects to burn). If an object is on fire, it will obviously transfer its heat energy to the surroundings!
Other examples are explosions and nuclear reactions, as these also release heat.
Inside your body, respiration in your cells is another example of an exothermic reaction.
Endothermic reactions
Endothermic reactions are the opposite of exothermic reactions.
An endothermic reaction is one that absorbs heat energy from the surroundings. This makes the surroundings become cooler and the object become warmer.
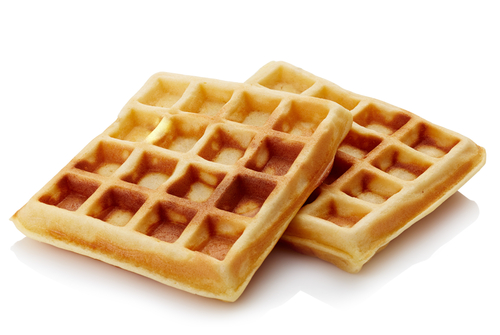
An example of endothermic reactions is cooking. The eggs, butter, flour, and milk all had to absorb heat energy from the oven to turn into the waffles shown above.
A natural example of an endothermic reaction is photosynthesis, as plants have to absorb heat from the Sun for the reaction to work.
.jpg)
Changes of state
You should be familiar with the changes of state shown below.
.jpg)
With the definitions of exothermic and endothermic in mind, we can now think about which changes of state match each type of process.
You will notice that condensation, freezing, and deposition are all drawn with blue arrows. This represents the object needing to be cooled down in order to experience those changes of state. As we saw earlier, if the object itself is cooling down, it means that it is transferring its heat energy to the surroundings. For this reason, these changes of state are exothermic.
The arrows in red - melting, freezing, and sublimation - all require the object to heat up, and this heat energy would have to be absorbed from its surroundings. Therefore, these three changes of state are endothermic.
Let's have a go at these questions now.
You can look back at this page by clicking on the red help button on the screen at any point.
.jpg)







