What is density?
Density is a measure of how close the particles are in a given volume of a substance. If the particles are very close together, we say that the material is more dense, and if they are far apart, it is less dense.
.png)
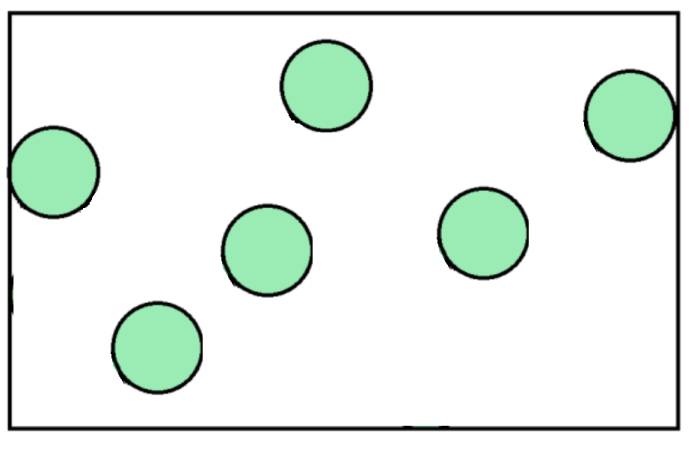
The diagrams above show a dense gas on the left, and a less dense gas on the right. Both gases are taking up the same space (which means they have the same volume), but the gas with more particles in this space has the greater density.
How do I calculate density?
There is an equation for density and it is:
Density = Mass / Volume
So, if you know the mass of a substance in kilograms, and its volume in m3, you just divide them by each other. This gives you a density in kg/m3
Sinking and floating
.jpg)
Have you ever wondered why things sink or float? The answer is density!
If you place something in a fluid, it will sink if its density is greater than the fluid, and it will float if its density is less than the fluid. This is the reason that a huge boat can float in the ocean. It may weigh several hundred tonnes, but it takes up such a large volume that its density is less than water. Meanwhile, something light, such as a coin, will sink because it is more dense than water.
So remember, it isn't the mass that determines whether something sinks or floats - it is the density.
Why is ice a bit weird?


You should know the arrangement of particles in solids, liquids, and gases. Using the idea of density, we can clearly see that solids have the greatest density, liquids the second most, and gases the least. When a substance changes state, you need to be aware that the density will also change due to the particles changing their spacing.
Let's finish by looking at ice and water, as these are unusual! As we have seen, solids have a greater density than liquids as their particles are closer together. This means that the solid form of a substance would sink when placed in its liquid form.

Ice breaks this rule, as when water freezes, its particles actually become further apart, and this means its density decreases. Therefore, ice floats in water.
That's a lot to remember, but you can look back at this page at any point by clicking on the red help button on the screen.







