As you probably know, solids, liquids, and gases are all made of particles. Their arrangements are shown below:
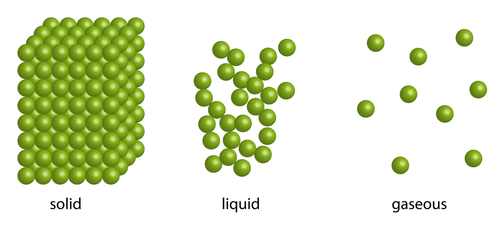
These are called the states of matter. In solids, the particles vibrate around fixed positions, while in liquids and gases ('fluids'), the particles are free to move and slide around. Gas particles move faster than solid and liquid particles. In all three states of matter, every particle is continuously moving.
How do particles behave differently with temperature?
If you heat a substance, you will give its particles more kinetic energy. This is the form of energy that depends on movement. The faster something moves, the more kinetic energy it has, and so increasing the temperature will have this effect on its particles.

Furthermore, the particles will move further apart from each other when the temperature increases. This happens in solids, liquids, and gases - but it is more noticeable in gases! Even solids will expand slightly when they are hotter, as the particles move slightly further away from their fixed positions as they vibrate. Gases will just spread further through the room if you take the lid off their container. (This process is called diffusion, but we don't need to go into detail on that here). Increasing the space between particles and making an object take up more space means that its volume will increase.

Finally, a property that changes with temperature is density. This is a measure of how close together the particles are, and so increasing the temperature will force the particles further apart, and therefore decrease the density. If you put food colouring into a liquid and then heat it, you will see parts of it floating and sinking due to these changes in density.
Now, test your understanding with some questions!








