Diffusion is the spreading out of particles. It can occur across membranes, into and out of cells and between different regions. Some examples of where diffusion takes place almost constantly is in the lungs, in a leaf, in the root hair cells of a plant and in liver cells.


It is the movement of particles down a concentration gradient, from a high concentration to a low concentration.
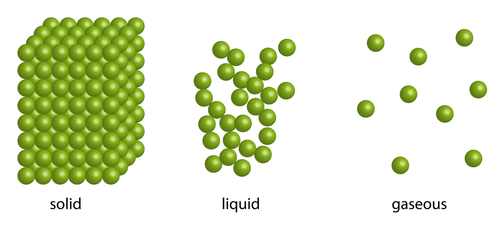
Diffusion takes place in liquids and gases very readily. It doesn't take place in solids however. This is because the particles in a solid do not move very much - they just vibrate on the spot, so particles have no space to wriggle their way through a solid to diffuse.
Can you speed up diffusion?
The rate of diffusion can be affected by temperature. The higher the temperature, the more kinetic energy the particles will have, so they will move faster and mix more quickly. Similarly, the lower the temperature, the less kinetic energy the particles will have so diffusion will happen much slower.
Have you got all that?
Then let's test out what you know in the following questions.







