How many different types of atoms are there?
Well, we know that there are 118 different types of atom because there are 118 different elements. An element is made from just one type of atom.
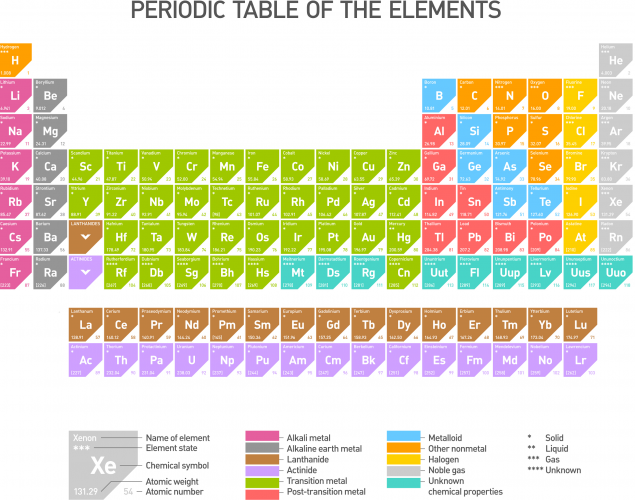
We find a list of all the elements that exist on the periodic table. Here, all the elements are arranged in a specific way, with similar elements grouped together.
Elements all have one thing in common - they are each made from only one type of atom. So gold is made from only gold atoms, mercury is made from just mercury atoms, and zinc is made from ...... you guessed it, zinc atoms!
.png)
But atoms of elements can join up with other types of atoms to form different substances.
When atoms of one element join together with other atoms of a different element, a compound is formed. Compounds are not found on the periodic table. Compounds contain different types of atoms chemically bonded together. This means that the atoms are bonded together really tightly, which makes separating them very difficult. In order for a compound to be separated, a chemical reaction must take place.
The properties of a compound are often very different to the properties of the elements it is made of.
Let's take iron sulfide as an example. Iron sulfide is a compound made from the elements sulfur, a yellow powder, and iron, a magnetic grey solid. The iron sulfide compound that is formed when sulfur and iron are heated strongly together, is a black solid but is not magnetic (unlike iron).

We can use particle diagrams to show the atomic structure of compounds.
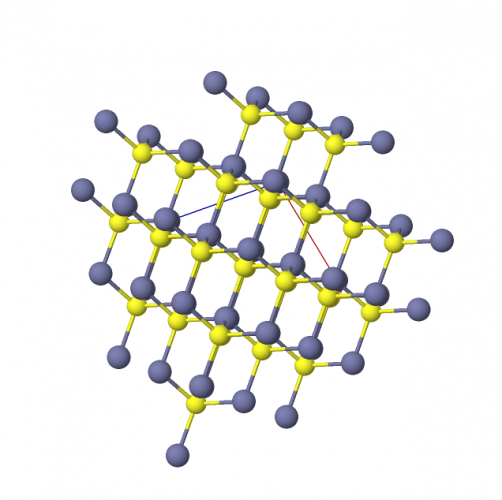
The molecule above is a a compound of a salt - it is a compound as it is made from two different elements, so two types of atoms. We know that they are different types of atoms as they are different colours.
Remember, a compound is very different to a mixture. A mixture consists of two or more substances, but they are not chemically bonded together. So separating a mixture can be done easily.
That's a lot of information about atoms, elements and compounds. Fancy trying some questions about these three? Let's go.....







